The Greek word ὀρείχαλκος and the corresponding Latin word orichalcum, otherwise spelled aurichalcum, designated different metals or alloys at different times, 1 but this Latin word and its equivalent in modern languages is now used by numismatists to designate a copper alloy containing zinc which the Romans employed in very late republican times and in imperial times as a material for coins. Some writers on Roman numismatics imply or state that orichalcum was an alloy of fixed composition, but this is not in accord with the facts. At no one time were the proportions of copper and zinc held exactly constant, and with passage of time the proportion of zinc gradually decreased while the proportions of tin and lead, which in the beginning were present in very small amounts as mere impurities, increased to such an extent that one or the other, or both, became important components of the alloy. Hence, in a strict sense, the term orichalcum should be understood to refer not to a single alloy but to a class of alloys that contained copper and zinc as principal components. Though Roman alloys of this class may be called brass, they contain lower proportions of zinc than most varieties of modern brass. Orichalcum is therefore a convenient and distinctive term for designating the particular kind of brass manufactured by the Romans. The complex copper alloys containing zinc, tin, and lead as principal components, used in the third century as materials for coins, should preferably be called zinc bronzes rather than orichalcum when the proportion of zinc is less than the proportion of tin, or when it is less than the proportion of tin and lead taken together.
It is not true, as has sometimes been stated, that orichalcum is the earliest known copper alloy containing zinc. Examples of much earlier alloys are now known. This study begins with an inquiry as to their place and time of origin, and a discussion of the difference between these alloys and orichalcum as to composition and probable method of manufacture.
The scanty information on the origin and manufacture of orichalcum given by ancient authors has been variously interpreted by modern writers. Often, isolated statements of single authors have been interpreted without regard to the body of information as a whole, and without regard to certain chemical or metallurgical facts. It seemed worthwhile to bring together in one place all the known ancient technical information about orichalcum, and to present some fresh interpretations of its significance.
Many false or misleading statements about the composition of orichalcum, and of orichalcum coins, exist in the literature of numismatics. Some of these statements are the result of the insufficient amount of chemical data available at the time, others the result of failure to make use of the data available, and still others the result of failure to interpret these data correctly. This study contains a critical account of all previous investigations of the composition of orichalcum coins, and presents the results of new and exact analyses of twenty-five such coins. On the basis of both the old and new data the changes in the composition of orichalcum with time of manufacture are traced in detail, and possible reasons for these changes are suggested. An attempt is made to establish the probable date when orichalcum ceased to be manufactured for the Roman coinage, and to determine the probable duration of the period when it was used only as a reworked alloy. The composition of late sestertii and dupondii struck in zinc bronzes or other alloys is discussed, especially from the standpoint of determining when orichalcum ceased entirely to be employed as a material for coins. Some consideration is given to the composition of small orichalcum objects other than coins, and the theory is advanced that coins served as the immediate source of the metal for many of these objects. The possibility of dating illegible orichalcum coins and other orichalcum objects from their chemical composition is also considered. Finally, a comparison is made between the composition of genuine orichalcum and that of a few modern forgeries of ancient objects composed of copper alloys containing zinc.
| 1 |
Rossignol, J. P., Les métaux dans l'antiquitié (Paris, 1863), pp. 205–331, deals in detail with the etymology of these words and their various meanings. The spelling orichalcum is definitely to be preferred to aurichalcum. For additional etymological information see: Diergart, P., Zeitschrift für angewandte Chemie, XIV (1901), pp. 1297–1301; ibid., XV (1902), pp. 761–763; ibid., XVI (1903), pp. 85–88. Disagreement with some of the conclusions
of Diergart was expressed by Neumann, B., Zeitschrift für angewandte Chemie, XV (1902), pp. 511–516, 1217–1218.
|
Although the Romans were undoubtedly the first to employ alloys of copper and zinc for coins, it is not true, as has sometimes been asserted, that this was the first use of such alloys for any purpose. Centuries before their use by the Romans for coins, copper alloys containing zinc were sporadically produced and used for the manufacture of a variety of objects. For a proper understanding of the distinctive nature of orichalcum, it is necessary to consider first the composition of these earlier alloys.
The comprehensive researches of Otto and Witter and their coworkers show that copper alloys containing moderate proportions of zinc were occasionally produced even in the Early Bronze Age in Central Europe. Of the more than thirteen hundred metal objects analyzed by these investigators, thirty were found to contain zinc as a component of the alloy. 2 Nine of their analyses of such objects are listed in Table I. These analyses were made by combination of chemical and spectrographic methods. By reason of the stated degree of accuracy of most of the determinations the results are here given only to one decimal place. The twenty-one other objects were analyzed solely by spectrographic methods, and though numerical results were not obtained for the copper content, the zinc content, or both, the analyses showed clearly that significant proportions of zinc were present. It will be seen that only one of the objects listed in Table I was found to contain more than twenty per cent. A low to moderate zinc content is characteristic of very early alloys of copper and zinc. No example has yet been found of such an alloy in which the proportion of zinc reaches a third, the usual proportion in modern yellow brass. It will also be seen from Table I that most of the objects contained tin in low proportions, and that a few of them contained significant proportions of lead. The presence of tin or lead, or both together, in a proportion not much inferior to that of the zinc is frequent in ancient copper alloys containing zinc, and sometimes the proportion of tin or lead, or both, considerably exceeds that of the zinc.
| Object No. | Copper % | Zinc % | Tin % | Lead % | Other Elements % | Total % |
| 1 | 75.0 | 22.6 | l.1 | 0.2 | 0.1 | 99.0 |
| 2 | 74.3 | 16.5 | 4.8 | 2.9 | 1.7 | 100.2 |
| 3 | 79.3 | 14.2 | 4.2 | 1.6 | 0.9 | 100.2 |
| 4 | 84.5 | 13.9 | 0.1 | 0.5 | 1.0 | 100.0 |
| 5 | 75.0 | 12.8 | 4.0 | 6.5 | 1.7 | 100.0 |
| 6 | 84.5 | 9.5 | 4.6 | 0.1 | 1.0 | 99.7 |
| 7 | 85.9 | 8.5 | 4.6 | 0.2 | 0.7 | 99.9 |
| 8 | 86.2 | 8.0 | 5.0 | 0.1 | 0.6 | 99.9 |
| 9 | 81.9 | 7.5 | 6.3 | 3.4 | 0.6 | 99.7 |
Analyses of various other prehistoric objects composed of copper alloys containing zinc are listed in Table II. At least some of these objects are later in date than those listed in Table I. No. 1 was analyzed by Moss 3 in the course of his investigations of the residues left in metallurgical crucibles used by the crannog dwellers of Ireland. In view of the small weight of metal available for analysis, his results were correctly expressed only to the first decimal place. The style of No. 2, analyzed by Church, 4 clearly indicated a pre-Roman date. Though he expressed his results to two decimal places, they are here given only to one in view of his high summation and his failure to determine certain impurities which must have been present. No. 3 was analyzed by Fellenberg. 5 This investigator found significant proportions of zinc in several other objects, but these appear to belong in the Roman period, though a few may be earlier. No. 4, analyzed by Helm, 6 is notable as containing an unusually high proportion of zinc for an ancient object. The presence of so much bismuth is also very unusual. Though there has been some controversy as to the date of this object, the most probable date appears to be the middle of the La Tène period. No. 5, also analyzed by Helm, is believed to belong to the earlier part of this same period. Nos. 6 to 9, inclusive, analyzed by Bibra, 7 all came from mound graves in the province of Hannover, Germany. Their exact age is uncertain, though they are probably all much earlier than the beginning of the Christian Era.
| Object No. | Copper % | Zinc % | Tin % | Lead % | Other Metals % | Total % |
| 1 | 78.8 | 15.5 | 3.1 | 0.9 | 1.9 | 100.2 |
| 2 | 88.2 | 9.1 | 3.6 | — | — | 100.9 |
| 3 | 80.30 | 16.31 | 2.85 | 0.16 | 0.38 | 100.00 |
| 4 | 63.86 | 30.62 | 1.13 | 0.18 | 4.21 | 100.00 |
| 5 | 70.71 | 27.30 | 1.04 | trace | trace | 99.05 |
| 6 | 75.70 | 19.05 | 3.14 | 0.88 | 1.86 | 99.63 |
| 7 | 82.85 | 12.87 | 3.02 | 0.93 | 0.33 | 100.00 |
| 8 | 87.19 | 9.70 | 1.01 | 0.70 | 1.40 | 100.00 |
| 9 | 87.05 | 5.00 | 7.86 | trace | 0.09 | 100.00 |
The earliest known metal objects of the Mediterranean region that contain significant proportions of zinc were found at Gezer in Palestine. 8 In most of these the zinc content is less than four per cent, and could be regarded as a mere accidental impurity. However, one of the objects from this site was found to contain 23.40 per cent of zinc, along with 10.17 per cent of tin and 66.40 per cent of copper. This object, the metal of which may be termed a zinc bronze, is believed to belong to the period known as Semitic III (1400–1000 b.c.). This is by far the earliest known object from the Mediterranean region in which zinc is clearly present as a principal component of the alloy. Moreover, no other object from this region dated prior to the first century b.c. has yet been found in which zinc is present with copper as a principal component of an alloy. Many bronze objects from the intervening period of about a thousand years have been analyzed, but zinc, when present at all, has been found to occur in these as an accidental impurity in proportions seldom exceeding a few tenths of one per cent. Bibra 9 found 2.30 per cent of zinc in a Macedonian bronze coin and 3.72 per cent in a bronze coin of Syracuse, but these are unusually high proportions for Greek bronze of any kind. The highest proportion of zinc found in any Greek bronze coin analyzed by the author and his co-workers was 0.20 per cent, and of a group of eighty such coins there were fifty in which zinc was not detected at all. 10
The only metal objects that may be classified as Greek and in which zinc has been found to be a principal component of the alloy were found at or near Greek colonies or settlements on the Black Sea. Only four objects of this kind are known. All were analyzed by Bibra. 11 The results of his analyses are listed in Table III. The reason for the perfect summations of all these analyses is that Bibra customarily determined copper by difference, i.e., by subtracting the total of all other determinations from 100.00 per cent in order to find the percentage of copper. On the basis of archaeological evidence all these objects are of late b.c. date. Nos. 1 and 2 are believed to belong in the first century b.c. Nos. 3 and 4 are dated before the beginning of the Christian Era, and probably also belong in the first century b.c. However, there is some possibility that the actual date of manufacture of some or all of these objects may be a little earlier than the first century. It will be seen that small proportions of tin are present in Nos. i and 2 along with the zinc. In this respect their composition resembles that of some of the prehistoric objects listed in Tables I and II. But the composition of Nos. 3 and 4 does not, since they are free from tin and contain very little lead. In being composed essentially of copper and zinc alone, the alloys of which these objects were made resemble the early type of orichalcum used by the Romans for coinage. They differ, however, in containing much lower proportions of zinc.
| Object No. | Copper % | Zinc % | Tin % | Lead % | Other Metals % | Total % |
| 1 | 82.76 | 13.31 | 3.40 | 0.19 | 0.34 | 100.00 |
| 2 | 84.87 | 10.12 | 4.36 | 0.21 | 0.44 | 100.00 |
| 3 | 91.00 | 9.00 | none | trace | traces | 100.00 |
| 4 | 90.59 | 8.10 | none | trace | 1.31 | 100.00 |
The first known use of alloys of copper and zinc for coinage occurred in the late Roman Republican period, apparently just after the middle of the first century b.c. This conclusion is based chiefly on the four analyses listed in Table IV. These analyses were published by Bahrfeldt, 12 who does not name the analyst. The figures given by Bahrfeldt indicate that only copper and zinc were determined, though the deficiencies in the summations of three of the analyses show that certain impurities, such as tin, lead, or iron, were also present. How- ever, since each of these deficiencies amounts to little more than one per cent, the individual proportions of the various other metals present as impurities in these coins must have been small. These four analyses are clearly very important from the standpoint of numismatic history, for they establish the time of the introduction of orichalcum as a coinage metal. They are the only quantitative chemical analyses that have been made of orichalcum coins issued before the reign of Augustus. However, one coin of the late Roman Republican period has been shown by a spectrographic test to consist chiefly of copper and zinc, the proportion of zinc being estimated to be in the range 10–15%. This coin was issued in Macedonia in 44 b.c., possibly by the quaestor, Acilius. 13 Slightly later coins, issued by Sosius at Zacynthus in 37 b.c., are said to be composed of orichalcum, and it has been suggested that a few other moneyers of the very late Republican period may also have issued coins in orichalcum, though decisive evidence based on chemical or spectrographic tests appears to be lacking. 14 On the other hand, such tests have clearly shown that a few types of late Republican or early Imperial coins, erroneously classed as orichalcum coins because of their color, are in fact composed of ordinary tin bronze or leaded tin bronze.
| Coin No. | Copper % | Zinc % | Total % |
| 1 | 71.11 | 28.88 | 99.99 |
| 2 | 71.10 | 27.60 | 98.70 |
| 3 | 78.60 | 20.30 | 98.90 |
| 4 | 83.84 | 15.00 | 98.84 |
Grant (From Imperium to Auctoritas, Cambridge, 1946, pp. 11, 63) is of the opinion that the coins of C. Clovius were issued in Cisalpine Gaul, probably at its capital, Mediolanum, and that those of Q. Oppius were issued in Syria, probably at Antioch and perhaps also at Apamea or Laodicea.
The copper alloys containing zinc, which appeared before orichalcum was regularly used for coinage by the Romans, differ in various respects from this Roman alloy. Their zinc content varies over a wide range, in the prehistoric alloys especially, as is shown by the data in Tables I and II. Moreover, as is shown in Table V, these earlier alloys contain higher proportions of metals other than copper and zinc. Especially significant are the higher proportions of tin and lead, which are present only in slight proportion as impurities in the orichalcum first used as a coinage metal by the Romans. Another important difference is the sporadic occurrence in respect both to place and time of objects composed of these earlier alloys, and their great scarcity as compared to objects of similar age composed of tin bronze. In contrast, orichalcum coins were regularly produced in a few localities in enormous numbers for nearly two centuries beginning with the time of Augustus. All these differences indicate that the earlier alloys were produced accidentally and that orichalcum was intentionally manufactured. A possible exception may be some of the copper-zinc alloys produced in the vicinity of the Black Sea, as seems to be indicated by the composition of the objects numbered 3 and 4 in Table III.
| Group No. | Kind and Source of Objects | Source of Data | Other Elements, % Maximum Average | |
| I. | Early Bronze Age, Central Europe | Table I | 12.2 | 6.5 |
| II. | Prehistoric, British Isles and Northern Europe | Table II | 8.0 | 4.5 |
| III. | Greek, Northern Black Sea Coast | Table III | 5.0 | 2.6 |
| IV. | Orichalcum Coins, Roman Republic | Table IV | 1.3 | 0.9 |
| V. | Orichalcum Coins, Roman Empire, b.c. Period | Table XVII | 1.2 | 1.0 |
These differences also indicate that the prehistoric alloys were produced by a somewhat different process than the one used for the manufacture of orichalcum. They could not have been made by the direct alloying of copper and zinc, as in modern practice, since metallic zinc could not have been produced in the open crucibles, crude furnaces, or primitive hearths used for smelting in prehistoric times. The metallurgy of zinc is peculiar in that the temperatures required to reduce its minerals or ores to metal with carbon are close to the boiling point of the metal itself, so that it vaporizes away as soon as it is formed unless some means is employed to condense or otherwise trap it. Furthermore, unless air is excluded, the vaporized metal burns immediately to its oxide at these elevated temperatures. Nor could these earlier alloys have been produced by the reduction of a mixture of copper and zinc ores, or a single ore containing both copper and zinc minerals. Bronze may be easily produced by an analogous process in primitive apparatus from a mixture of copper and tin ores, but brass cannot be produced by this general method for making alloys because a temperature high enough to reduce the copper and zinc ores would also be high enough to vaporize and oxidize practically all the zinc before it could alloy with any copper formed by reduction. However, under certain conditions copper containing a low percentage of zinc may sometimes have been accidentally made. If a heterogeneous mixture of the two ores and charcoal were unevenly heated in a crucible some copper might have been formed by reduction before all the zinc ore was reduced. Some of the zinc from the reduction of the remaining zinc ore might then have been trapped by the metallic copper before it could vaporize. This is one way to account for the presence of the low proportions of zinc that not infrequently occur as an impurity in prehistoric copper. More than a few per cent could not have been introduced into copper by this means.
The formation of a copper-zinc alloy by cementation appears to be the only way by which the prehistoric alloys were produced. In this method, as it was carried out in early modern times, thin bars or small pieces of copper are buried in a mixture of zinc ore and charcoal contained in a crucible. On heating the crucible and its charge to a sufficiently high temperature some of the zinc formed by reduction is vaporized and lost but most of it is trapped in the hot surface of the copper to form a copper-zinc alloy. By the subsequent fusion of the metal, and by stirring, a homogeneous alloy is produced. This was the method employed for making brass in early modern times, and in some places it was used long after metallic zinc became generally available for making brass by direct alloying. In prehistoric times the production of a copper-zinc alloy in this way was in all probability the accidental result of one of the processes then commonly used for the manufacture of bronze. In this process crude copper obtained by the reduction of a copper ore with charcoal, or even wood, was heated with a mixture of tin ore and fuel. 15 The tin formed by the reduction of the tin ore alloyed with the copper, and by fusion and stirring a homogeneous alloy was produced. Probably the prehistoric bronze makers at the period when this process was in use did not even recognize that tin was formed as a separate metal, but believed rather that the process was one in which the crude copper was improved in quality by being treated in this way. In the early development of the process various minerals and ores were in all probability tried before tin ore, in the form of cassiterite or tinstone, was found to be the one that gave the best results. Even after this became well known it is probable that other ores were tried as substitutes or extenders when tin ore became unavailable or scarce. Moreover, the superficial resemblance of some other ores to tin ore may have caused them to be used unintentionally. 16 Thus it came about that at various places and times some of the easily reduced ores of zinc and lead were sometimes substituted for tin ore to a greater or lesser extent with the result that the alloy obtained by this process contained tin, zinc, and lead in various combinations and in a wide range of random proportions. As shown in Tables I and II this is just what the analyses show for prehistoric copper alloys that contain zinc as a component. Moreover, the variety and proportions of the various minor components or impurities in these alloys indicate clearly the use of many different kinds of ores.
| 2 |
Otto, H., and Witter, W., Handbuch der ältesten vorgeschichtlichen Metallurgie in Mitteleuropa (Leipzig, 1952), pp. 210–211.
|
| 3 |
Moss, R. J., Proceedings of the Royal Irish Academy, XXXVII C (1924–1927), p. 186.
|
| 4 |
Church, A. H., Journal of the Chemical Society, XVIII (1865), p. 216.
|
| 5 |
Fellenberg, L. R. von, Mitteilungen der Naturforschenden Gesellschaft in Bern (1863), p. 54.
|
| 6 |
Helm, O., Zeitschrift für Ethnologie, XXVII (1895), pp. 7–8.
|
| 7 |
Bibra, E. von, Die Bronzen und Kupferlegierungen der alten und ältesten Völker (Erlangen, 1869), pp. 122–123.
|
| 8 |
Macalister, R. A. S., The Excavations of Gezer
(London, 1912), Vol. II, pp. 265, 293, 303. The analyses were made by J. E. Purvis.
|
| 9 |
Bibra, E. von, op.cit., pp. 86–87
|
| 10 |
Caley, E. R., The Composition of Ancient Greek Bronze Coins (Philadelphia, 1939), p. 151.
|
| 11 |
Bibra, E. von, op.cit., pp. 98–99, 102–103
|
| 12 |
Bahrfeldt, M., Numismatische Zeitschrift, XXVII (1905), p. 42.
|
| 13 |
Grant, M., From Imperium to Auctoritas (Cambridge, 1946), pp. 17, 18, 89, 493.
|
| 14 |
Grant, M., op.cit., pp. 40, 89.
|
| 15 |
Forbes, R. J., Metallurgy in Antiquity (Leiden, 1950), p. 250.
|
| 16 |
Prehistoric miners and metallurgists probably experienced great difficulty in distinguishing clearly certain of the darkcolored
ores from each other. For example, tinstone and zinc blende sometimes resemble each other closely in both color and luster.
No such difficulty was experienced, however, in recognizing the oxidized ores of copper which they used for bronze manufacture
since these have a very striking blue or green color.
|
Any inquiry into the origin and technology of orichalcum must involve the question of whether ancient metallurgists ever produced metallic zinc, and if so, whether they recognized it as a distinct metal. Though the discovery of ancient objects composed of zinc has at various times been reported by archaeologists, the stated identification as zinc appears often to have depended on physical appearance rather than on chemical analysis. The earliest such report was by Grignon, 17 who examined a piece of metal found at a Roman site between St. Dizier and Joinville in France and concluded that it was worked zinc. But his reasons for this identification are vague, and probably no reliable chemical tests were made, if indeed they could have been made at such an early date. Salzman 18 reported that certain bracelets found at the ancient necropolis of Kameiros on the Island of Rhodes were formed of hollow silver filled with zinc. He does not state that chemical tests were made, and the possibility exists that he may have based his identification on the grey crystalline appearance of internally corroded silver. Moser 19 reported that an ancient bell-shaped object covered with a mottled bluish-grey and yellowish-brown patina, found in excavations at Castelvenere near Trieste, was composed of zinc or a zinc-antimony alloy. He also fails to state that any chemical tests were made, and the uncertainty of his identification seems to indicate that he depended solely on the appearance of the patina. A few other vague or uncertain identifications have been reported.
Helm 20 was the first to show clearly by chemical analysis that an ancient metal object was composed mostly of zinc. This object, described by him as being a prehistoric Dacian idol, is stated to have been found at Tordos in Transylvania. It was covered with a bluish-grey layer of corrosion products. On testing the object with a file the metal was seen to be white. Helm at first thought that the metal was antimony, but he found on analysis that a sample of it had the following composition:
| Metal | % |
| Zinc | 87.52 |
| Lead | 11.41 |
| Iron | 1.07 |
On the basis of these results Helm concluded that the metal was crude zinc. He sent a sample to the famous anthropologist, Virchow, who, because of the apparent historical importance of the identification, had the sample examined independently by another chemist. This chemist found that the sample was not a uniform alloy, as the results obtained by Helm would seem to indicate, but was composed of two layers soldered or welded together, the one being zinc and the other lead. However, the essential fact was confirmed that metallic zinc was the principal component. In his criticisms of the conclusions of Helm, Virchow 21 pointed out that the object had not been found under the controlled conditions of archaeological excavation, but was evidently a surface find, and that therefore its real place of origin and its actual date were unknown. He even questioned the authenticity of the object, though Helm had previously stated that fraud or forgery was out of the question. Virchow concluded that there was no evidence to show that it was a prehistoric Dacian object, though he admitted that it could have been made in Dacia during the Roman period. In a later paper, Helm 22 announced that he had identified another ancient Dacian object as being composed mostly of zinc. This object, from a private collection of antiquities, was an elongated lump of corroded metal with an iron wire imbedded in it, which seemed to indicate that it might have been the clapper of a bell. Unfortunately, the provenance of this object was also uncertain.
The only ancient specimen of definitely known provenance identified beyond doubt as metallic zinc was found in the course of the excavation of the Agora at Athens. 23 As to provenance, the discoverer, Dr. Arthur W. Parsons, reported as follows: 'The fragment of zinc was found in Section OA on May 13,1939, at the base of the cliff on the north slope of the Acropolis, at a point about 7.0 meters east of the ancient fountain house, the Klepsydra, and directly below the cave sanctuary of Pan. The pottery and coins with which it was found were chiefly of the 4th and 3rd centuries b.c.; there was nothing later than the 2nd century b.c. It may be regarded as certain that the zinc got there no later."
The fragment was in the form of a piece of flat sheet, roughly rectangular in shape, and measuring about 65 by 40 mm. Most of it was 0.50 to 0.55 mm. thick, but it was very thin at the edges. A few pits penetrated right through the piece. The fragment was at first supposed to be composed of lead, and it was not until after it has been drastically cleaned to see if it bore an inscription that qualitative tests were made that revealed that it was composed mostly of zinc. Half of the fragment was sent to the Research Laboratory of the New Jersey Zinc Company for quantitative spectrographic analysis. The following results were reported for the principal metallic impurities present:
| Metal | % |
| Lead | 1.3 |
| Cadmium | 0.060 |
| Iron | 0.016 |
| Copper | 0.0055 |
Smaller proportions or traces of magnesium, manganese, antimony, tin, and silver were also found. This is a greater variety of impurities than is present in modern zinc, but the proportion of lead is less than in some grades of modern zinc, and the proportions of cadmium and iron are no higher.
A metallographic examination of a sample of the fragment showed that the metal had the structure of slightly worked zinc, but not the structure of sheet zinc formed by the modern method of rolling. Metal of the structure observed could have been formed by hammering out a lump of cast zinc not more than ten times thicker than the fragment.
The puzzling feature about this fragment is its survival in such a location for so long a period. Concerning this, Farnsworth, Smith, and Rodda state: "While it is hard to believe that a sample of a reactive metal like zinc would not disappear by corrosion in much less than 2000 years in a location damp enough to cause severe corrosion of adjacent bronze coins, the spectrographic and metallographic analyses agree in showing that the present sample is unlike modern zinc in both composition and method of working, and support the archaeological evidence of its antiquity." However, they suggest that this fragment of zinc had a protective surface layer or a coating that retarded its corrosion. Unfortunately, the opportunity of demonstrating the presence of such a layer or coating and of ascertaining its nature was lost because the fragment was so drastically cleaned before its unique nature was recognized.
Because of the chemical reactivity of the metal, the great scarcity of ancient zinc objects might conceivably be ascribed to their general disappearance through corrosion. However, if metallic zinc really had been abundant and in general use in Greek and Roman times, it seems likely that many zinc objects, particularly those of thick metal, would have survived in protected locations. Perhaps the strongest argument against the abundance or general use of metallic zinc in classical antiquity is the moderately low zinc content of copper-zinc alloys that have survived from that period. In not one of the considerable number of objects composed of such alloys that have been analyzed does the zinc content reach forty per cent, and generally it is much lower. Such a low limit to the zinc content of these alloys indicates that they were made by a cementation process and not by the direct alloying of the two metals. If metallic zinc had been generally available for alloying with copper, it is highly probable that ancient metallurgists would have manufactured some alloys of higher zinc content and that some objects composed of such alloys would have survived.
In general, the archaeological evidence shows that metallic zinc was extremely scarce in classical antiquity. Probably it was only accidentally and occasionally made in very small amounts in a few places in the course of certain metallurgical operations, probably in much the same way as zinc was first accidentally isolated in small amounts in certain metallurgical operations in Europe in early modern times. According to Rickard 24 the metal was not recognized in modern Europe until 1509 when it was detected in the form of beads or drops in slag at the silver-smelting works on the Rammelsberg in Germany. A little later it was found in small amounts as metal condensed in cracks or crevices in the walls of certain smelting furnaces. 25 In view of the composition of the primary ore worked for silver in the famous Greek mining district of Laurion in ancient times, it seems very likely that zinc was sometimes isolated in the slag or smelting furnaces during the long period when these mines were worked. This Laurion ore is a mixture of the sulfides of iron, lead, and zinc. 26 The silver is contained in the lead sulfide (galena) and the ancient Greeks isolated this from the mixture of sulfides by a sorting and washing process before the actual smelting. 27 However, it is likely that this separation was often imperfect and that some zinc sulfide (sphalerite) was included in the refined ore smelted for silver. In fact, there is evidence for this in a statement by Pliny 28 who says that a product called lauriotis, evidently a form of zinc oxide named after the mining district at Laurion, was formed in silver furnaces. In another place 29 he remarks that cadmea unquestionably occurs in furnaces used for silver smelting. This term, when applied to an artificial product, undoubtedly designated zinc oxide formed on the walls of smelting furnaces. 30 From other remarks made by Pliny 31 it may be inferred that the size and design of these furnaces were such that considerable quantities of zinc oxide and other useful volatile products collected on their walls and in their chambers. This makes it seem even more likely that small amounts of metallic zinc sometimes condensed in cracks or crevices of the walls of these furnaces.
In the text the word χαλκός is used to denote the metal that is like gold in appearance. Usually this has been translated as copper, but, since it is obviously yellow in color, bronze is a much better translation. There is no justification for translating as brass merely on the basis of color since polished bronze of proper tin content may also resemble gold or certain gold alloys rather closely in color. Moreover, in another part of this same work (sec. 58) a distinction is made by the use of different words between the metal of a certain bronze statue and the metal of others made of oreichalkos. After some remarks on the copper deposits of the island of Demonesus, the writer goes on to say: ἔστι δὲ αὐτόθι χαλκὸς κολυμβητὴς ἐν δυοῖν ὀργυιαῖς τῆς θαλάσσης ὅθεν ὁ ἐν Σικυῶνί ἐστιν ἀνδριὰς ἐν τῷ ἀρχαίῳ νεῷ τοῦ Ἀπόλλωνος καὶ ἐν Φενεῷ οἱ ὀρείχαγκοι καλούμενοι. (There is also copper to be dived for in two fathoms of sea. From this is made the statue in Sicyon in the ancient temple of Apollo, and those in Pheneus called oreichalkos statues.) From the wording it might seem that these statues were composed of the copper itself, but in view of the fact that Greek statues generally were made of alloyed copper, the correct meaning must be that the copper was used in making the alloys for these statues. It may also be noted that no archaeological evidence exists for the use of brass or zinc bronze in India or Persia at the time of Darius.
The second account in the work On Marvelous Things Heard, which merits more serious and extensive consideration, comprises all of sec. 62, and reads as follows: Φασὶ τὸν $$οσσύνοικον χαλκὸν λαμπρότατον καὶ λευκότατον εἶναι, οὐ παραμιγνυμένου αὐτῷ κασσιτέρου, ἀλλὰ γῆς τινὸς αὐτοῦ γινομένης καὶ συνεψομένης αὐτῷ. λέγουσι δὲ τὸν εὑρόντα τὴν κρᾶσιν μηδένα διδάξαι διὸ τὰ προγεγονότα ἐν τοῖς τόποις χαλκώματα διάφορα, τὰ δ᾽ ἐπιγιγνόμενα οὐκέτι. (They say that the bronze of the Mossynoeci is very shiny and light in color, though tin is not mixed with the copper, but a kind of earth which occurs there is smelted with it. But they say that the discoverer of the mixing process did not instruct anyone else, so that the bronze objects formerly produced there are superior, whereas those made subsequently are not.) The first phrase is usually translated thus: (They say that the copper of the Mossynoeci is very shiny and light in color, though tin is not mixed with it.) Although this may have been the meaning the writer of the account intended to convey, a pale or light-colored metal could not have been copper itself, but must have been an alloy of copper. The word χαλκός may mean either copper or bronze, and bronze seems the better translation. However, from the standpoint of grammar the reflexive pronoun at the end of the phrase then appears to refer back to bronze, and this poses a difficulty from the standpoint of metallurgy, for obviously tin would not be added to bronze but to copper. As indicated by the translation this difficulty may be resolved by assuming that the reflexive pronoun refers back to χαλκός in its alternate meaning of copper.
The word λευκότατον used in describing the appearance of the alloy may be translated as very white instead of very pale or very light in color. If this word is so understood, then the alloy could not have been ordinary bronze or brass. Tin bronzes containing more than 25 per cent of tin are white and so are brasses containing more than 75 per cent of zinc, though such alloys are too brittle for most practical uses. But there is another possibility. It could have been a copper-arsenic alloy, for such an alloy, even when it contains a relatively small proportion of arsenic, is also white. Alloys of copper with arsenic, which may be called arsenic bronzes, were known in the Aegean region from very early times, as has been shown by chemical analyses of certain prehistoric metal objects. 61 Moreover, the existence in ancient times of the practice of whitening copper by treating it with arsenic minerals, or with products derived from such minerals, is shown by two recipes in the Leyden Papyrus X. One of these (No. 23) reads as follows:
Χαλκᴏῦ λεύᴏσις
Χαλκὸν λευκὸν πᴏιῆσαι, ὣστε µειγέσθαι ἀσήμῳ, ἲσᴏν ἲσῳ, καὶ ἀνένκλητᴏν εἶναι. λαβὼν χαλκὸν Κὺπριᴏν, χεὺνευσᴏν, βαλὼν εἰς τὴν μνᾶν σανδαράχης, τῆς σαπρᾶς, τῆς σιδριζᴏὺσης ∠β᾽, καὶ στυπτηρίας σχιστῆς ∠ε᾽, καὶ χώνενε. Τῇ δευτέρᾳ χωννύσει βάλλεται κηροῦ Ποντικοῦ ∠δ᾽, εἰ μὴ, καὶ πυροῦται καὶ ῾ρήσσεται 62
(Whitening of Copper
To whiten copper, so that it can be mixed with silver bullion in equal parts, and not be recognized: take Cyprian copper, melt it, having added for each mina 2 drachmas of decomposed irony realgar and 5 drachmas of lamellose alum, and cast it. For the second fusion 4 drachmas of Pontic wax are added; if not, it is burnt by the fire and breaks.)
Probably the decomposed realgar mentioned in this recipe was obtained by roasting the natural mineral, possibly under reducing conditions which would produce a mixture of arsenious oxide and arsenic having a grey color. The alum perhaps served as a flux. Both the roasted arsenic mineral and the alum would have anciently been classified as earths. The wax probably prevented oxidation of the copper-arsenic alloy. One objection to this recipe as a parallel to the account in the work On Marvelous Things Heard is the discrepancy in time. The Leyden Papyrus X was probably compiled in the third century a.d., whereas the account was probably written in the third century b.c., and the wording would appear to indicate that the process itself was even earlier. However, many of the recipes in the papyrus undoubtedly represent technical processes that had been in use for a very long period. Whether any credence can be placed in the statement in the second sentence of the account as to the loss of the knowledge of the process is perhaps doubtful since the mention of lost arts is a favorite device for adding interest to stories.
In view of the possibility that the bronze of the Mossynoeci may have been a copper-arsenic alloy, it cannot be unquestionably assumed, as so many have done, that the earth mentioned in this account is calamine and that the alloy produced without tin is brass. Nevertheless, there is a considerable probability that the account does allude to the manufacture of brass by the cementation process, and that it is the earliest known allusion to this process. What seems to support this view is that the only known Greek objects composed of brass or zinc bronze (Table III) have been found at or near the sites of colonies or settlements on the Black Sea, near the shore of which, in Pontus, dwelt the people known as the Mossynoeci. Even if this account in the work On Marvelous Things Heard does not allude to a process for making brass, it is still important, as is likewise the one in the treatise On Stones of Theophrastus, for explaining how the manufacture of brass by the cementation process came to be discovered. Once the practice of heating various mineral substances with copper came into use, it was inevitable that sooner or later zinc ores would be heated with the metal in the presence of a reducing agent and brass would be produced. What remains unknown, and probably always will remain unknown, is exactly where and when this discovery took place. Largely on the basis of the account just discussed, Forbes 63 believes that the discovery took place in Pontus, but the evidence seems too incomplete to establish this with certainty. Forbes is also of the opinion that the discovery took place in the first half of the first millenium b.c., but real evidence for such an early date appears to be lacking. The available literary evidence does not indicate a date any earlier than the fourth century b.c., and the archaeological evidence does not indicate a date any earlier than the second century b.c. at the earliest. It was not until after the middle of the first century b.c. that the intentional manufacture of brass by the cementation process began on any considerable scale, and its manufacture by this process on a continuous and large scale did not begin until after the foundation of the Roman Empire.
The essential difference in composition between the alloys produced before the middle of the first century b.c. and those produced later has already been pointed out. Another distinct difference is in their use. The earlier alloys were never used for coinage, whereas the orichalcum of the Romans was used chiefly for coinage, and indeed at the beginning and for a considerable time afterwards this appears to have been its only use. Our knowledge of the composition of orichalcum must therefore be derived chiefly from analyses of Roman Imperial coins composed of this alloy.
| 17 |
Grignon, P. C., Bulletin des fouilles d'une ville romaine (Bar-le-Duc, 1774), p. 11.
|
| 18 |
Salzman, A., Revue archeologique, IV (1861), p. 472.
|
| 19 |
Moser, L. K., Mitteilungen der anthropologischen Gesellschaft in Wien, XXXV (1905), "Sitzungsberichte," p. 52.
|
| 20 |
Helm, O., Verhandlungen der Berliner Gesellschaft für Anthropologie, Ethnologie und Urgeschichte (1895), p. 621.
|
| 21 |
Virchow, R., Zeitschrift für Ethnologie, XXVIII (1896), pp. 338–339.
|
| 22 |
Helm, O., Correspondenz-Blatt der deutschen Gesellschaft für Anthropologie, Ethnologie und Urgeschichte, XXX (1899), p. 100.
|
| 23 |
Farnsworth, M., Smith, C. S., and Rodda, J. L., Hesperia, Supplement VIII (1949), PP. 126–129.
|
| 24 |
Rickard, T. A., Man and Metals (New York, 1932), Vol. I, p. 158.
|
| 25 |
Lohneys, G. E., Bericht vom Bergwercken (Zellerfeldt, 1617), pp. 83–84.
|
| 26 | |
| 27 |
Some of the ancient sorting and washing basins and tables have remained essentially intact at Laurion up to the present time.
|
| 28 |
Natural History, Book XXXIV, sec. 132.
|
| 29 |
Natural History, Book XXXIV, sec. 100.
|
| 30 |
Bailey, K. C., The Elder Pliny's Chapters on Chemical Subjects (London, 1929–1932), Part II, pp. 166–167.
|
| 31 |
Natural History, Book XXXIV, secs. 101–102.
The great scarcity of metallic zinc in classical antiquity is also indicated by the scarcity of information about it in the
works of the writers of that period. Only two passages, both by Greek authors, can be interpreted as referring to the isolation
of zinc, and one of these is apparently a fairly close quotation of the other. The original passage occurred in the Philippica of Theopompus, a historian of the fourth century b.c., but its occurrence in this work is known to us only through quotations of it or references to it in the works of much later
writers. It is, for example, quoted by Stephen of Byzantium, a writer of the sixth century a.d. in one of the surviving fragments of his Geographical Lexicon.
32
This writer also indicates that an earlier quotation of it is given by Strabo in his Geography, which was written about the beginning of the Christian Era. Strabo, himself, does not state or even hint that he quoted
from Theopompus, but a comparison of the two quotations clearly shows that he did. The quotation by Strabo
33
may be closer to the original, for he was much nearer to Theopompus in time of writing and probably used an earlier manuscript
of the Philippica than was available to Stephen of Byzantium. The text of the pertinent part of the passage in Strabo is as follows:ἔστι δὲ
λίθος περὶ τὰ Ἄνδειρα ὃς καιόμενος σίδηρος γίνεται εἶτα μετὰ γῆς τινὸς καμιυευθεὶς ἀποστάζει ψευδάργυρον, ἣ προσλαβοῦσα χαλκὸν
τὸ καλόμενον γίνεται κρᾶμα, ὅ τινες ὀρείχαλκον καλοῦσι
Although the language of this text is fairly clear, its meaning from the metallurgical standpoint is rather obscure, and chiefly
for this reason it has been translated and interpreted in various ways. Some scholars have even altered the standard text
of the passage in order to improve its sense, but without sound justification. The following is a close translation: There
is a stone near Andeira which yields iron when burnt. After being treated in a furnace with a certain earth it yields drops
of false silver. This, added to copper, forms the so-called mixture, which some call oreichalkos.
This passage is obviously a sketchy account of a metallurgical process by which a copper alloy called oreichalkos was produced. At
the time of Strabo there seems to be no question that the Greek ὀρείχαλκος was the equivalent of the Latin orichalcum and that both meant an alloy of copper and zinc. Hence it would seem evident that the Greek ψευδάργυρος (false silver) must
be metallic zinc. Most scholars have concluded that this was the process, or one of the processes, in use for the production
of orichalcum around the beginning of the Christian Era. What they have overlooked, however, is the strong probability that
Strabo has here quoted blindly from Theopompus and that he had no first-hand knowledge of this process, which may not have
even been in use in his day. It is almost certain that Strabo never traveled in the part of Asia Minor where Andeira was located.
34
On this basis alone, he could not have had a first-hand knowledge of the process. Moreover, he gives no indication anywhere
in his Geography that he possessed any understanding of metallurgy. Hence this passage does not constitute evidence that orichalcum was made
by the direct alloying of copper and zinc at the time of Strabo. It would seem rather to indicate that the alloy was made
in this way at the time of Theopompus or earlier, and that the Greek word ὀρείχαλκος also meant an alloy of copper and zinc
at this earlier period in spite of the lack of archaeological evidence that brass was known to the Greeks in the fourth century
b.c. or earlier.
Various Greek writers, some earlier and some later than Theopompus, mention ὀρείχαλκος as a particular metal or alloy, usually
in a way that indicates that it was one of considerable rarity and value. The earliest such mention is in the anonymous Shield of Herakles,
35
almost certainly composed prior to 500 b.c., in which the poet says,
36
Ὥ εἰπὼν κνημῖδας ὀρειχάλκιο φαεινοῦ Ἡφαίστου κλυτὰ δῶρα, περὶ κνήμῃσιν ἔθηκεν. (So he spoke, and placed about his legs his
greaves of shining oreichalkos, the glorious gift of Hephaistos.) Somewhat later is a passage in one of the Homeric hymns to Aphrodite
37
in which the anonymous poet relates that the Hours attached to the ears of the goddess an ornament of precious gold and orichalcum.
The text
38
reads in part: καλὴν χρυσείην, ἐν δὲ τρητοῖσι λοβοῖσιν ἄνθεμ’ ὀρειχάλκου χρυσοῖό τε τιμήεντος. Plato in his Critias, probably written near the middle of the fourth century, refers to the metal in several passages
39
in his description of the mythical island of Atlantis. In describing the walls around the capital he says: τοῦ δ’ ἐντὸς καττιτέρῳ
περιέτηκον, τὸν δὲ περὶ αὐτὴν τὴν ἀκρόττολιν ὀρειχάλκῳ μαρμαρυγὰς ἔχοντι πυρώδεις. (They covered the interior wall with fused
tin, and the wall around the acropolis itself with oreichalkos, which has a fiery resplendence.) As to the temple of Poseidon on the Acropolis he relates that: Πάντα δὲ ἔξωθεν περιήλειψαν
τὸν νεὼν ἀργύρῳ, πλὴν τῶν ἀκρωτηρίων, τὰ δὲ ἀκρωτήρια χρυσῷ τὰ δ’ ἐντός, τὴν μὲν ὀροφὴν ἐλεφαντίνην ἰδεῖν πᾶσαν χρυσῷ καὶ
ἀργύρῳ καὶ ὀρειχάλκῳ πεποικιλμένην, τὰ δὲ ἄλλα πάντα τῶν τοίχων τε καὶ κιόνων καὶ ἐδάφους ὀρειχάλκῳ περιέλαβον. (All the outside
of the temple, except the pinnacles, they covered with silver, but the pinnacles with gold. As to the interior, the roof was
covered entirely with ivory variegated with gold, silver and oreichalkos. All the rest, the walls, columns and pavement they ornamented with oreichalkos.) Further on he relates that the laws of the first kings were engraved on a column of this metal: ἐν στήλῃ γεγραμμένα ὀρειχαλκίνῃ
In his discussion of the metals available to the inhabitants of Atlantis, Plato states that, except for gold, oreichalkos was the most precious metal which existed at that time: πλήν χρυσοῦ τιμιώτατον ἐν τοῖς τότε ὄν
Since it is listed along with gold and silver as a metal of great value, it might reasonably be concluded that the oreichalkos of these writers could not have been brass. But if zinc was very scarce at this period, as the archaeological evidence appears
to indicate, and if the alloy was made in the way Theopompus states, then brass would have been so rare that it could have
had a value above that of silver, which, at that time, was being obtained in abundance from the mines of Laurion. Rossignol
40
advanced the theory that the oreichalkos of these early Greek writers was a mythical metal that had no real existence, an invention of the poets. He based this theory
in part on another statement made by Plato in his Critias, where Plato says that oreichalkos was something now known only by name, but formerly was something
more than a name: καὶ τὸ νῦν ὀνομαζόμενον μόνον τότε δὲ πλέον ὀνόματος ἦν But this statement may mean merely that the alloy
was not in use at the particular time when Plato wrote, or that it was in use without Plato being aware of it. Possibly he
was not aware of it because it was no longer used locally.
The exact nature of the metallurgical process so briefly described by Theopompus has been the subject of much controversy,
and has almost invariably been treated as though the account was original with Strabo. However, the question of its true authorship
does not affect the problem of its interpretation from the metallurgical standpoint. This problem may conveniently be discussed
by a systematic examination of the meaning of the words, phrases and sentences of the account. The translation previously
given will be used as a basis, but any additional or alternate meaning conveyed by the Greek text will also be considered.
"There is a stone near Andeira which yields iron when burnt." The Greek word λίθος, here translated as stone, had a much broader
meaning than is indicated by this usual English equivalent, for it included any hard substance of mineral origin. Here the
context clearly shows that some metallic mineral or ore is meant. Andeira was the name of a town in northwestern Asia Minor,
but its exact site is unknown.
41
Any correlation of its location with known mineral deposits or ancient mining sites is therefore impossible. The verb here
translated as burnt might perhaps be better translated as smelted since it seems probable that the process involved the reduction
of some mineral or ore that contained iron, although the suggestion has been made that it was a simple roasting process which
yielded a product that merely had the superficial appearance of iron,
42
the basis of this suggestion being that no reducing agent is mentioned. However, the metallurgists of the period could recognize
iron with certainty since this metal was being regularly produced on a considerable scale. Hence reduction must have been
one of the steps in the process. Very likely the need for mixing the ore with charcoal in order to obtain iron in a smelting
operation is not explicitly mentioned be-
cause this was such a common practice. However, it seems almost certain, from the kind of ore that was apparently used, that
the first step in the whole process was the roasting of the ore, and that no reducing agent was added in this step. The identity
of this ore is not entirely certain, though the remainder of the account would seem to indicate that it was one that contained
zinc as well as iron. The only common ore that meets this requirement is zinc blende, impure zinc sulfide, which usually contains
a considerable proportion of iron. Diergart
43
was of the opinion that zinc blende was unlikely because no mention is made of the choking sulfur dioxide fumes that would
have resulted from roasting or smelting it. However, the writer of the account may not have been aware of this, or may simply
have not considered it worth mentioning. A roasting operation would have been necessary as a first step in order to convert
zinc blende to a form suitable for reduction, i.e., to a mixture of iron and zinc oxides. If a reducing agent were then added
and the smelting done without special apparatus or precautions, the zinc would have been lost and only iron would have been
obtained. In other words, if this stone found in the vicinity of Andeira was smelted in the ordinary way only iron could be
obtained from it. But the writer of the account apparently goes on to state how a second metal could be obtained from this
same ore by a special treatment. However, an alternate interpretation is that the stone was a simple iron ore and that the
second metal was obtained only because a different ore was added in the second step.
"After being treated in a furnace with a certain earth it yields drops of false silver." The identity of the earth with which
the roasted ore was treated has been the subject of controversy. The Greek word γῆ included any kind of friable or soft material
that occurred in the ground, or any artificial product of this sort obtained by treating mineral substances in various ways.
Here it would appear to be a natural product. Both Rossignol
44
and Diergart
45
suggested that it was a flux of some kind. Helm
46
suggested that it was ordinary coal or
an earthy coal which acted as a reducing agent, and Leaf
47
identified it as lignite, but Diergart
48
regarded such identifications as doubtful because he believed that any form of coal would have been designated by its usual
generic name. Moreover, no reducing agent for obtaining iron from the ore is mentioned in the first step of the process, and
it seems likely that the use of a reducing agent is also implicit here. If the stone of Andeira was an iron ore, the earth
mentioned here must have been a zinc ore of some kind. The most likely possibility, because of its common occurrence in the
general area, is calamine. The main objection to its identification as calamine is that this ore usually occurs in the form
of a rather hard compact mineral which Greek writers would call a stone rather than an earth, but perhaps it was calamine
that had been refined by roasting to drive out the combined water, and then pulverized. Such preliminary treatment would have
been almost necessary for the successful smelting of this ore. On grammatical grounds, the objection to the identification
of the earth as calamine in any form is that it is the stone of Andeira, not the earth, which is said to yield the false silver,
but this statement could well be based on a lack of understanding of the chemistry of the process by the ancient metallurgists
or by the writer of the account. Some significance may be attached to the fact that this earth is alluded to in such a vague
way, without a specific name and without a mention of its source. Possibly, in their desire to preserve secrecy, the metallurgists
deliberately withheld this information, as well as other essential facts, from the writer of the account. This same tendency
to secrecy in regard to what may have been the same mineral substance appears to occur in at least one later Greek account
in which the author fails to identify by either name or place of origin a particular earth used in making a copper alloy which
may have been brass.
Whatever may have been the exact identities of the ores and other raw materials used in the process, and whatever may have
been the various essential but unmentioned working details, there seems to be no doubt that the false silver produced in the
second step was more or less pure zinc. It must have been a white metal that resembled silver but was not silver, and in all
probability it also was not tin or
lead since either would have been given its usual name. This leaves zinc as the only possibility among the metals known in
Greek times. Diergart
49
was of the opinion that pure zinc could not have been produced in this process in view of the likelihood that the reduction
to zinc could not have occurred without the simultaneous formation of iron. He concluded that the product was an alloy of
the two metals containing a few per cent of iron. However, such impure zinc would have served almost as well as pure zinc
for alloying with copper in the last step of the process. There is also the possibility that the zinc was separated by distillation.
The Greek verb ἀποστάζει in the text appears to imply the production of the zinc in drops, or drop by drop, which at least
hints at a distillation process. Leaf
50
interprets the passage in this sense by translating as follows: Near Andeira there is a stone which when calcined becomes
iron; and then, when treated in the furnace with a certain earth, distills mock silver. Helm
51
suggested that the reduction was carried out in a vessel with a tight fitting cover, and that the vessel was also provided
with an outlet tube in the bottom into which the zinc vapor passed and condensed, finally to emerge as drops of molten metal
from the bottom of this condenser tube. Though this kind of apparatus has been used for the production of zinc in modern times,
no archaeological or literary evidence exists for the use of any such apparatus in ancient times. However, it might have been
possible to condense the zinc in drops on the walls and flues of a furnace of special design if a large excess of charcoal
was used for the reduction, though the efficiency of the operation would probably have been low because of the loss of much
of the zinc through oxidation. If the metal had been obtained by distillation it would, of course, have been purer than any
metal obtained without such separation.
"This, added to copper, forms the so-called mixture, which some call oreichalkos." The metal to which the zinc was added may
have been bronze rather than copper, for χαλκός here translated as copper is a generic term that included at the time of this
account any metal that
had the general appearance of copper. Hence it included ordinary bronze as well as copper itself. Indeed, since bronze was
more commonly used for most purposes, the word more often denotes this alloy than unalloyed copper. Only when a modifying
adjective is used is there any certainty as to which is meant. Hence the alloy formed by fusion with zinc could have been
either brass or zinc bronze, and consequently oreichalkos could mean either one.
Though κρᾶμα means mixture in general, it usually means mixed wine, and less often a medicinal mixture. Its use here to denote
a mixture of metals is quite exceptional, and this appears to be indicated also by the use of καλόμενον (so-called). Perhaps
κρᾶμα is here a technical term that would be better translated as mixed metal. Its translation as amalgam by Leaf
52
is not satisfactory since this term is now generally reserved for an alloy that contains mercury as one of its components.
From the standpoint of the history of zinc and its alloys with copper this account by Theopompus is important not only because
it contains the earliest mention of a process for the isolation of this metal but because it also contains the earliest mention
of the manufacture of an alloy containing zinc and copper. No other ancient author except Strabo, who merely repeats the account
of Theopompus, describes any process that can be interpreted as involving the direct alloying of zinc with copper or bronze.
This method of making brass or zinc bronze apparently never was used either continuously or on a large scale in ancient times.
According to all the evidence, none but the cementation process was employed when orichalcum came to be manufactured regularly
on a large scale in Roman Imperial times.
Certain accounts in the works of Greek writers of the Hellenistic period have often been cited as containing allusions to
the manufacture of brass, but unfortunately these accounts are even more ambiguous and sketchy than the one by Theopompus.
The earliest of these Hellenistic accounts is contained in the treatise On Stones by Theophrastus of Eresos written near the end of the fourth century b.c. This account reads as follows: ἰδιωτάτη δὲ ἡ τῷ χαλκῷ μιγνυμένη πρὸς γὰρ τῷ τήκεσθαι καὶ μίγνυσθαι καὶ δύνμιν ἔχει περιττὴν
ὥστε τῷ κάλλει τῆς χρόας ποιεῖν διαφοράν. (The most unusual
earth is the one mixed with copper; for in addition to melting and mixing, it also had the remarkable power of improving the
beauty of the color.)
53
Among those who assume without question that this account refers to the manufacture of brass by the calamine cementation
process are Rossignol,
54
Rickard,
55
and Forbes.
56
The validity of this uncritical conclusion will now be examined briefly.
In the first place it is uncertain that ordinary copper is the metal that was treated with the earth. In another section of
this same treatise, where Theophrastus describes the preparation of verdigris by the action of sour grape residues on copper,
he uses the term χαλκὸς ἐρυθρὸς (red copper) for the metal that was employed, which clearly indicates that unalloyed copper
is meant. The lack of this qualifying adjective in the above account makes it appear likely that the metal treated with the
earth was bronze rather than copper. In the second place there is no evidence of any kind that the earth was calamine, or
any other kind of zinc ore. It could have been any one of a number of earthy minerals. The existence in ancient times of the
practice of melting metals with various mineral substances of this kind is shown by the many recipes in the Leyden Papyrus
X that describe this practice explicitly.
57
Some of these substances were arsenic minerals, such as realgar, which served to whiten the copper through the formation
of a copper-arsenic alloy, others were clayey minerals which served to exclude air from the surface of the metal on fusion
and thus prevent oxidation, and still others were bituminous materials which served to reduce metal oxides on fusion and produce
clean metal. There are two indications that the unusual earth in this account was a bitumen of some sort. One is the melting
of the earth and the other is the mention by Theophrastus in the very next sentence following this account of another peculiar
earth that was undoubtedly a natural bituminous substance.
58
Though it is possible
that Theophrastus in this account is alluding to the manufacture of brass or zinc bronze, it seems much more probable that
he is alluding to a mere refining process in which bronze, in the form of crude or scrap metal, was melted with a natural
bituminous material in order to obtain clean metal of improved appearance.
Also frequently cited as evidence for the manufacture of brass in Greek times are two accounts in the pseudo-Aristotelian
work On Marvelous Things Heard, a compilation usually attributed to students or successors of Aristotle, and believed to have been written for the most
part in the third century b.c., though some passages of this work are evidently based on earlier sources. The following account, which comprises all of
sec. 49, is apparently based, at least in part, on such sources: Φασὶ δὲ καὶ ἐν Ἰνδοῖς τὸν χαλκὸν οὔτως εἶναι λαμπρὸν καὶ
καθαρὸν καὶ ἀνίωτον ὥστε μὴ διαγινώσκεθαι τῇ χρόᾳ πρός τὸν χρυσόν, ἀλλ’ ἐν τοῖς △αρείου ποτηρίοις βατιακὰς εἶναί τινας καὶ
πλείους, ἃς εἰ μὴ τῇ ὀσμῇ, ἄλλως οὐκ ἦν διαγνῶναι πότερόν εἰσι χαλκαῖ ᾓ χρυσαῖ. (They also say that among the Indians the
bronze is so bright, clean and free from corrosion that it is indistinguishable in appearance from gold, but that among the
cups of Darius there is a considerable number which could not be distinguished as bronze or gold except by the odor.)
On the basis of this account Partington
59
remarks that, "Brass was probably made in Persia in the Achaemenian Period, since Darius is said to have had a bowl like gold in appearance but distinguishable by its unpleasant
smell." Forbes
60
appears to follow Partington in part when he asserts that, "In Iran brass came into use in the Achaemenian Period. Darius
is said to have possessed an 'Indian' cup which looked like gold but had a disagreeable smell, which points to brass." Certain
inaccuracies are apparent in these partial paraphrases, but the main point is that neither the tentative remark of Partington
nor the positive assertion of Forbes can be justified on the basis of this account. There is even the possibility that this
account may be an exaggerated or fanciful traveler's tale, but even when taken literally it provides no real evidence for
the use of brass in either India or Persia at the time of Darius.
|
| 32 |
For the text of this quotation see: Grenfell, B. P., and Hunt, A. S., Hellenica Oxyrhynchia cum Theopompi et Cratippi Fragmentis (Oxford, 1909), Philippica, Book XIII, sec. 109.
|
| 33 |
Geography, Book XIII, sec. 56.
|
| 34 |
Leaf, W.,
Strabo on the Troad (Cambridge, 1923), pp. xxviii–xxxviii.
|
| 35 |
Usually included in editions of the works of Hesiod.
|
| 36 |
v. 121–122.
|
| 37 |
VI, v. 8, 9.
|
| 38 |
According to Allen, T. W., Halliday, W. R., and Sikes, E. E., The Homeric Hymns (Oxford, 1936), p. 73.
|
| 39 |
Secs. 114 e, 116 b, d, 119, c–d.
|
| 40 |
Rossignol, J. P., Les métaux dans l'antiquité (Paris, 1863), pp. 214–236.
|
| 41 |
Leaf, W.,
Strabo on the Troad (Cambridge, 1923), pp. 284–287.
|
| 42 |
Helm, O., Verhandlungen der Berliner Gesellschaft für Anthropologie, Ethnologie und Urgeschichte (1895), pp. 622–623.
|
| 43 |
Diergart, P., Journal für praktische Chemie, CLXXV (1903), pp. 326–334, 429–432.
|
| 44 |
Rossignol, J. P., Les métaux dans Vantiquité (Paris, 1863), p. 253.
|
| 45 |
Diergart, P., Journal für praktische Chemie, CLXXIV (1902), p. 343.
|
| 46 |
Helm, O., Verhandlungen der Berliner Gesellschaft für Anthropologie, Ethnologie und Urgeschichte (1895), p. 623.
|
| 47 |
Leaf, W.,
Strabo on the Troad (Cambridge, 1923), p. 289.
|
| 48 |
Diergart, P., Journal für praktische Chemie, CLXXV (1903), pp. 330–331.
|
| 49 |
Diergart, P., Journal für praktische Chemie, CLXXIV (1902), pp. 339–345.
|
| 50 |
Leaf, W.,
Strabo on the Troad (Cambridge, 1923), p. 284.
|
| 51 |
Helm, O., Verhandlungen der Berliner Gesellschaft für Anthropologie, Ethnologie und Urgeschichte (1895), p. 623.
|
| 52 |
Leaf, W.,
Strabo on the Troad (Cambridge, 1923), p. 284.
|
| 53 |
Text and translation according to Caley, E. R., and Richards, J. F. C.,
Theophrastus On Stones (Columbus, 1956), pp. 26, 55.
|
| 54 |
Rossignol, J. P., Les métaux dans l'antiquité (Paris, 1863), p. 254 (footnote).
|
| 55 |
Rickard, T. A., Man and Metals (New York, 1932), p. 157.
|
| 56 |
Forbes, R. J., Metallurgy in Antiquity (Leiden, 1950), p. 278.
|
| 57 |
Berthelot, M., Archéologie et histoire des sciences (Paris, 1906), pp. 268–283, 290–291, 296–299.
|
| 58 |
Caley, E. R., and Richards, J. F. C.,
Theophrastus On Stones (Columbus, 1956), pp. 167–169.
|
| 59 |
Partington, J. R., Origins and Development of Applied Chemistry (London, 1935), p. 410.
|
| 60 |
Forbes, R. J., Metallurgy in Antiquity (Leiden, 1950), p. 279.
|
| 61 |
Caley, E. R., Hesperia, Supplement VIII (1949), pp. 60–63.
|
| 62 |
Text of Berthelot, M., Archéologie et histoire des sciences (Paris, 1906), p. 278.
|
| 63 |
Forbes, R. J., Metallurgy in Antiquity (Leiden, 1950), pp. 279–280.
|
The first analyses of orichalcum coins, which, indeed, were the first quantitative analyses of brass objects of any kind, were made by the celebrated pioneer analytical chemist Martin Heinrich Klaproth. Though he announced the results of his investigation in a paper entitled "Mémoire de numismatique docimastique" read before the Royal Academy of Sciences and Belles-Lettres of Berlin on July 9, 1795, the first publication 64 of these results was delayed until 1798. The results of his analyses as he reported them are shown in Table VI. These same results calculated on a percentage basis are shown in Table VII. As might be expected from the state of numismatic knowledge in his day, his descriptions of the coins he analyzed are imperfect, though they are adequate for approximate identification as indicated in Table VI. His method of analysis, which he had to devise for the purpose, was inadequate by present standards and could not yield
| Coin No. | Copper Grains | Zinc Grains | Tin Grains | Lead Grains | Iron Grains | Total Grains |
| 1 | 119 | 31 | – | – | – | 150 |
| 2 | 187 | 46 | – | – | – | 233 |
| 3 | 296 | 84 | – | – | – | 380 |
| 4 | 293 | 59 | 3 | 4 | 1 | 360 |
| 5 | 326 | 53 | 3 | – | – | 382 |
| 6 | 294 | 60 | 11 | – | – | 365 |
Descriptions as Given by Klaproth
Obv.: Castor and Pollux as two horsemen, with the inscription CAESAR AUGUSTUS GERMANICUS.
Rev.: S C in center with illegible inscription.
Wt. = 150 grains [9.7 grams].
Coin of Nero and Drusus, sons of Germanicus.
Obv.: Quadriga.
Rev.: An indistinct figure which originally represented either an upright soldier or a trophy.
Wt. = 233 grains [15.1 grams].
Obv.: Head of Tiberius Claudius.
Oblong counterstamp behind head.
Rev.: Civic crown with inscription
EX S C OB CIVES SERVATOS.
Wt. = 380 grains [24.6 grams].
Obv.: Head of Vespasian.
Rev.: Soldier seated.
Wt. = 360 grains [23.3 grams].
Obv.: Head of Trajan.
Rev.: Seated figure, possibly Vesta.
Wt. = 382 grains [24.8 grams].
Obv.: Head of Trajan.
Rev.: Same as No. 5.
Wt. = 365 grains [23.7 grams].
After the publication of the analyses of Klaproth, no results of any investigation of the composition of orichalcum coins were published for half a century except the results of an analysis of a single coin by Göbel. 65 He described this coin as follows:
Obv.: Tiberius Claudius Caesar.
Rev.: Bust of Antonia Augusta.
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Total % |
| 1 | 79.3 | 20.7 | — | — | — | 100.0 |
| 2 | 80.3 | 19.7 | — | — | — | 100.0 |
| 3 | 77.9 | 22.1 | — | — | — | 100.0 |
| 4 | 81.4 | 16.4 | 0.8 | 1.1 | 0.3 | 100.0 |
| 5 | 85.3 | 13.9 | 0.8 | — | — | 100.0 |
| 6 | 80.6 | 16.4 | 3.0 | — | — | 100.0 |
This was evidently a dupondius of Claudius struck in a.d. 41. Göbel reported that the metal of this coin was composed of 72.20% copper and 27.7% zinc. These results must be regarded as only approximate, for he found no other metals and his method of analysis was not very good. Another coin, which Göbel evidently took to be Roman, was also analyzed and found to contain 10.5% zinc. However, his description indicates that this coin was a modern forgery. Göbel also analyzed a number of brass antiquities found in the Russian Baltic provinces, a few of which were probably of Roman origin. The composition of these is discussed later.
A small series of orichalcum coins was analyzed by Phillips. 66 His results, recalculated on the basis of present atomic weights from his analytical data, are shown in Table VIII. Though these results are probably more accurate than those obtained by Klaproth or Göbel, they are by no means free from error as is indicated by the unsatisfactory summations of two of the analyses. His imperfect descriptions and the corresponding identifications are also shown in the table. In addition to these four coins, Phillips analyzed a sestertius of Faustina Junior, which he found to contain a higher proportion of lead than zinc and almost as much tin as zinc. The metal of this coin should properly be classed as a zinc bronze.
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Total % |
| 1 | 82.38 | 17.36 | — | — | 0.36 | 100.10 |
| 2 | 81.07 | 17.82 | 1.05 | — | — | 99.94 |
| 3 | 83.13 | 15.90 | — | — | 0.51 | 99.54 |
| 4 | 85.77 | 10.89 | 1.15 | 1.74 | 0.75 | 100.30 |
Large Brass of the Cassia Family. About 20 b.c.
Wt. = 365 grains [23.7 grams].
Large Brass of Nero. a.d. 60. Rev.: Rome seated.
Wt. = 435 grains [28.2 grams].
Titus, a.d. 79.
Wt. = 178 grains [11.5 grams].
Hadrian, a.d. 120. Fortunae reduci.
Wt. = 365 grains [23.7 grams].
Among a considerable number of ancient objects and materials analyzed by Giradin 67 was a single Roman orichalcum coin which he described as having on one side a bust of Antonia Augusta and on the other the inscription Titus Claudius Caesar Augustus Imperator. The improbable reading "Titus" was apparently based on the first two letters which in all probability should be interpreted as "Tiberius". On the basis of this interpretation the coin was probably a dupondius of Claudius struck in a.d. 41. Giradin found that the coin contained 81.4% copper and 18.6% zinc. No other metals were reported and no great accuracy can be ascribed to his results.
Genth 68 analyzed a coin of Trajan and one of Hadrian. Since he gave neither weights nor descriptions, the denominations and exact dates of these coins are unknown. His results are shown in Table IX. No. 1 was the coin of Trajan and No. 2 the coin of Hadrian. Though his analyses are more complete than those of his predecessors, their accuracy is not high as is shown by the high summations.
| Coin No. | Copper | Zinc % | Tin % | Lead % | Iron % | Silver % | Total % |
| 1 | 88.58 | 7.56 | 1.80 | 2.28 | 0.29 | 0.21 | 100.72 |
| 2 | 86.92 | 10.97 | 0.72 | 1.10 | 0.18 | 0.30 | 100.19 |
The results obtained by Genth have had a curious history of careless citation by later writers. Bibra 69 in one place wrongly ascribed the first set of results to Phillips, and in another place he ascribed both sets to himself. Moreover, Bibra interchanged the names of the emperors. Stohman and Kerl 70 ascribed both sets of results to Pöpplein. Apparently these writers did not consult the original publication.
The most extensive early series of chemical analyses of orichalcum coins was carried out by Bibra. 71 His results are listed in Table X. Unfortunately, the coins he analyzed cannot be closely dated since he gave only the names of the emperors and the weights of the coins. The denominations of the coins shown in the table have been decided from these weights. The perfect summations of his analyses arise from the fact that he determined copper by difference, i.e., he subtracted the percentages of all the other components from 100.00% in order to obtain the percentage of copper. This means that the accumulated errors of all the other determinations fell on the copper which resulted in a corresponding error in the determination of this element. His determinations of some of the other elements may also be somewhat in error. For example, it is certain from his method of analysis and from the results of later analyses that his stated percentages of nickel are generally too high. In spite of these uncertainties, the analyses of Bibra provide much valuable information.
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Nickel % | Antimony % | Total % |
| 1 | 92.57 | 5.15 | 1.05 | trace | 1.03 | 0.20 | trace | 100.00 |
| 2 | 87.05 | 11.80 | 0.72 | trace | 0.43 | trace | trace | 100.00 |
| 3 | 77.44 | 21.50 | 0.30 | trace | 0.32 | 0.24 | 0.20 | 100.00 |
| 4 | 88.19 | 10.23 | 0.51 | 0.30 | 0.55 | 0.22 | none | 100.00 |
| 5 | 86.30 | 12.94 | 0.52 | trace | 0.14 | 0.10 | none | 100.00 |
| 6 | 82.13 | 15.35 | 1.12 | trace | 1.00 | 0.40 | trace | 100.00 |
| 7 | 83.95 | 12.42 | 2.22 | 0.30 | 0.39 | 0.50 | 0.22 | 100.00 |
| 8 | 78.24 | 20.23 | 0.70 | 0.13 | 0.40 | 0.30 | none | 100.00 |
| 9 | 90.76 | 5.12 | 3.22 | 0.70 | 0.13 | trace | 0.27 | 100.00 |
| 10 | 91.24 | 7.14 | 0.32 | 0.44 | 0.52 | 0.34 | none | 100.00 |
| 11 | 88.50 | 9.05 | 1.27 | 0.30 | 0.35 | 0.43 | 0.10 | 100.00 |
| 12 | 82.91 | 15.57 | 0.60 | 0.06 | 0.70 | 0.08 | 0.08 | 100.00 |
| 13 | 82.35 | 16.84 | 0.43 | trace | 0.38 | trace | trace | 100.00 |
| 14 | 89.92 | 6.74 | 1.52 | 0.37 | 1.15 | 0.30 | none | 100.00 |
| 15 | 90.49 | 7.04 | 1.10 | 0.20 | 1.07 | 0.10 | trace | 100.00 |
| 16 | 91.72 | 5.33 | 1.55 | trace | 1.30 | trace | trace | 100.00 |
| 17 | 87.88 | 11.28 | none | 0.09 | 0.37 | 0.38 | none | 100.00 |
| 18 | 87.86 | 8.14 | 3.86 | trace | 0.12 | trace | trace | 100.00 |
| 19 | 81.47 | 10.30 | 6.62 | 0.02 | 0.01 | 0.28 | 1.30 | 100.00 |
| 20 | 85.63 | 6.07 | 4.62 | 2.00 | 1.07 | 0.40 | trace | 100.00 |
| 21 | 90.28 | 5.90 | 2.00 | 0.41 | 0.91 | 0.28 | 0.22 | 100.00 |
| 22 | 87.70 | 7.92 | 2.90 | 0.42 | 0.73 | 0.31 | 0.02 | 100.00 |
| 23 | 85.60 | 5.77 | 4.02 | 4.17 | 0.13 | 0.21 | trace | 100.00 |
Sestertius of Augustus. Wt. = 23.7 grams.
Also contains a trace of cobalt and a trace of sulfur.
Sestertius of Augustus. Wt. = 23.4 grams.
Also contains a trace of arsenic.
Sestertius of Claudius. Wt. = 24.9 grams.
Also contains a trace of sulfur.
Dupondius of Domitian. Wt. = 10.2 grams.
Also contains a trace of cobalt and a trace of sulfur.
Dupondius of Nerva. Wt. = 11.6 grams.
Sestertius of Trajan. Wt. = 17.3 grams.
Also contains a trace of sulfur.
Sestertius of Hadrian. Wt. = 21.2 grams.
Dupondius of Hadrian. Wt. = 13.7 grams.
Also contains a trace of sulfur.
Sestertius of Hadrian. Wt. = 19.5 grams.
Also contains a trace of cobalt and a trace of arsenic.
Sestertius of Sabina. Wt. = 20.0 grams.
Also contains a trace of sulfur.
Sestertius of Antoninus Pius. Wt. = 24.0 grams.
Also contains 0.10% of sulfur.
Sestertius of Marcus Aurelius. Wt. = 18.1 grams.
Also contains traces of cobalt, silver and sulfur.
Sestertius of Marcus Aurelius. Wt. = 17.9 grams.
Also contains a trace of sulfur.
Dupondius or possibly an As of Lucius Verus.
Wt. = 10.7 grams. Also contains a trace of sulfur.
Sestertius of Commodus. Wt. = 17.6 grams.
Also contains a trace of arsenic.
Sestertius of Commodus. Wt. 22.3 grams.
Also contains 0.10% sulfur.
A sestertius of Julia Soaemias, one of three late sestertii analyzed by Hofmann, 72 was found to be composed of an alloy that may be classed as orichalcum, for the zinc content was 17.08%, the tin content only 4.00%, and the lead content only o.95%.The composition of this coin is considered in more detail under the discussion of the composition of late sestertii and dupondii.
Helm 73 analyzed a small series of orichalcum coins. The results of his analyses are shown in Table XI. He gave a date range for the coins of Vespasian and Marcus Aurelius that coincides with the period for the reign of these emperors and the range a.d. 98–112 for the two coins of Trajan. Since he gave no descriptions, the coins cannot be more closely dated. Nor does he state their denominations. These have been decided from the weights. The perfect summations of his analyses indicate that he determined one of the components by difference, probably the copper.
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Nickel % | Silver % | Total % |
| 1 | 85.89 | 13.02 | 0.40 | 0.31 | 0.17 | none | 0.21 | 100.00 |
| 2 | 80.09 | 15.45 | 2.28 | 1.63 | 0.15 | 0.40 | trace | 100.00 |
| 3 | 87.12 | 9.90 | 2.13 | 0.48 | 0.20 | none | 0.17 | 100.00 |
| 4 | 87.31 | 7.08 | 4.02 | 0.83 | 0.42 | 0.34 | none | 100.00 |
Grueber 74 published three analyses by Gowland of coins of Augustus, the results of which are shown in Table XII. The descriptions given by Grueber are also shown, except for the dates he assigned to the coins. For No. 1 he assigned the range 27–19 b.c., and for the other two he assigned the date 9 b.c. Later authorities assign different dates. Grant 75 is of the opinion that coins of the type of No. 1 were struck in 17 b.c. and later, and Mattingly 76 assigns the date 22 b.c. to coins of the types of Nos. 2 and 3. Since only the main components of the alloys were determined, and then only through the first decimal place, these analyses should be regarded as only approximate.
| Coin No. | Copper % | Zinc % | Tin % | Total % |
| 1 | 78.7 | 20.6 | 0.7 | 100.0 |
| 2 | 76.7 | 23.3 | none | 100.0 |
| 3 | 76.4 | 23.6 | none | 100.0 |
Descriptions
Dupondius of Augustus struck in the East.
Obv.: AVGVSTVS. Head of Augustus to r.
Rev.: C · A within laurel wreath ornamented with prows.
Sestertius of Augustus struck in Rome.
Obv.: OB Cl VIS SERVATOS. Oak wreath between two laurel branches.
Rev.: C · ASINIVSC · F · GALLVS IIIVIR · A · A · A · F · F. In center, S · C.
Wt. = 387.2 grains [25.1 grams].
Dupondius of Augustus struck in Rome.
Obv. : AVGVSTVS TRIBUNIC · POTEST. within wreath.
Rev.: C · ASINVS GALLVS IIIVIR · A · A · A · F · F. In center, S · C.
Wt. = 203.3 grains [13.2 grams].
One of a group of sixteen Celtic coins analyzed by C. Virchow and co-workers was evidently composed of orichalcum, though it is described as a bronze coin by Forrer 77 in his publication and discussion of the analytical results. This coin, listed as No. 10 in the published table of analyses, bore a head in imitation of that of Augustus, the representation of a bull, and the single word INDVTILLI. Forrer ascribed this so-called bronze coin to Germanus-Indutilli fili and remarked that it was similar to the one shown in Figure 175 of his comprehensive treatise on Celtic coinage. 78 Coins of this type were struck at Treviri and are believed to belong to the latest period of issue of the Celtic coinage of Gaul. The results of the analysis are shown in Table XIII.
| Metal | % |
| Copper | 81.68 |
| Zinc | 16.46 |
| Tin | 0.10 |
| Lead | 1.25 |
| Iron | 0.22 |
| Nickel | 0.24 |
| Silver | 0.05 |
| Total | 100.00 |
This is the only known example of an orichalcum coin not of official Roman issue. Perhaps other coins of the same type had a similar composition, and possibly late Celtic coins of other types were sometimes struck in orichalcum. It seems very probable that earlier or contemporaneous Roman orichalcum coins were the source of the metal for such coins.
The analyses of orichalcum coins published by Mattingly 79 exceed in number those published by any one writer up to the present time. 80 These analyses were executed mostly in the laboratory of the British Museum and at the Royal Mint. Though Mattingly names the analysts, or at least the persons responsible for the execution of the analyses, he does not state which analyses were done by particular individuals. These analyses may be divided into two distinct groups, one in which the zinc content of the coins was actually determined and the other in which the zinc content was merely estimated. The results of the first group are shown in Table XIV and those of the second group in Table XV. As shown by the figures for the individual determinations and for the summations, the quality of the analyses of the first group varies greatly. Most of the determinations are expressed only through the first decimal place, which is indicative of the ap- proximate nature of these determinations. Three of the summations are grossly deficient, which may indicate that oxidized coins were taken for analysis, that one or more of the individual determinations were inaccurate, or that components were present which were not determined. The six perfect summations are too many to be attributed to chance, and indicate the estimation of some component by difference. As far as the reported figures show, No. 8 is the only coin that was satisfactorily analyzed. The analyses of the second group are admittedly incomplete, though the proportions of copper, tin and lead in the coins appear to have been accurately determined. A closer estimate of the zinc content of the coins of this group seems possible by subtracting the sum of the percentages of copper, tin and lead of each analyses from 99.5%, a conservative estimate of the summation that would have been obtained if the analyses had been completed. The figures obtained in this way are shown in the fourth column of Table XV. Since no descriptions were given of the coins that were analyzed, no close estimate of their dates is possible. In spite of their various defects, many of the analyses published by Mattingly provide useful information.
| Coin No. | Copper % | Zinc % | Tin % | Lead | Iron % | Total % |
| 1 | 76.85 | 21.33 | — | 0.20 | 1.62 | 100.00 |
| 2 | 81.1 | 15.7 | — | — | 3.2 | 100.0 |
| 3 | 79.5 | 16.6 | trace | 1.3 | — | 97.4 |
| 4 | 78.08 | 16.68 | 2.14 | 0.57 | trace | 97.47 |
| 5 | 82.2 | 16.5 | 0.5 | 0.8 | — | 100.0 |
| 6 | 83.4 | 16.4 | — | trace | — | 99.8 |
| 7 | 84.8 | 14.8 | trace | trace | — | 99.6 |
| 8 | 85.14 | 13.98 | 0.68 | 0.12 | trace | 99.82 |
| 9 | 86.5 | 13.5 | — | — | — | 100.0 |
| 10 | 86.1 | 13.4 | 0.2 | — | — | 99.7 |
| 11 | 85.7 | 13.6 | — | — | — | 99.3 |
| 12 | 83.7 | 12.7 | 2.8 | 0.8 | — | 100.0 |
| 13 | 86.41 | 13.59 | — | trace | — | 100.00 |
| 14 | 86.81 | 7.96 | 1.41 | 1.30 | — | 97.48 |
| Coin No. | Copper % | Stated Zinc Content % | Zinc Content by Difference % | Tin % | Lead % |
| 1 | 86.39 | Over 12.0 | 13.1 | — | trace |
| 2 | 86.85 | Over 12.0 | 12.6 | — | trace |
| 3 | 92.79 | Over 7.0 | 6.7 | trace | trace |
| 4 | 88.71 | Over 10.0 | 10.8 | trace | trace |
| 5 | 88.59 | Over 10.0 | 10.4 | trace | 0.48 |
| 6 | 89.13 | Over 6.0 | 7.0 | 3.33 | trace |
| 7 | 87.73 | Over 5.0 | 7.5 | 1.58 | 2.71 |
| 8 | 87.47 | Over 5.0 | 5.4 | 5.06 | 1.56 |
| 9 | 78.24 | Over 10.0 | 10.2 | 3.12 | 7.98 |
| 10 | 87.07 | Over 6.0 | 7.1 | 1.94 | 3.37 |
| 11 | 82.69 | Over 6.0 | 5.8 | 2.93 | 8.12 |
Among the many Roman coins of the early empire examined spec-trographically in the laboratory of the British Non-Ferrous Metals Research Association at the request of Grant, 81 seven were found to contain enough zinc to be classed as orichalcum coins. However, these analyses were at best semi-quantitative, and no exact figures for the proportions of zinc, or for the proportions of the other components of the alloys were obtained. Though such analyses may be very useful for the classification of coins, they contribute nothing toward an exact knowledge of the composition of ancient coinage alloys.
Very few of the orichalcum coins which have been analyzed in the past have been adequately described or closely dated, and at least half of the chemical analyses are inaccurate, incomplete, or both. No example exists of a fully described coin that has been carefully analyzed. Obviously, what is needed for an exact knowledge of the composition of orichalcum, and especially for an exact knowledge of the changes in its composition with time, are satisfactory chemical analyses of a long series of adequately described and closely dated coins.
| 64 |
Klaproth, M. H., Mémoires de l'académie royale des sciences et belles-lettres, Berlin, Classe de philosophie expérimentale (1798), pp. 97–113. A German version of this same paper was later published in Sammlung der deutschen Abhandlungen, welche in der königlichen Akademie der Wissenschaften zu Berlin vorgelesen wurden in
den Jahren 1792–1797, Experimental-Philosophie (1799), pp. 3–14, under the title "Beitrag zur numismatischen Docimasie," and still later under the same title there appeared
a modified German version in Allgemeines Journal der Chemie, VI (1801), pp. 227–244.
|
| 65 |
Göbel, F., Über den Einfluβ der Chemie auf die Ermittelung der Völker der Vorzeit oder Resultate der chemischen Untersuchung metallischer
Alterthümer (Erlangen, 1842), p. 29.
|
| 66 |
Phillips, J. A., Journal of the Chemical Society, IV (1852), pp. 252–300.
|
| 67 |
Giradin, J., Journal für praktische Chemie, LX (1853), p. 92.
|
| 68 |
Genth, F. A., Journal of the Franklin Institute, XXXVI (1858), p. 266.
|
| 69 |
Bibra, E. von, Die Bronzen und Kupferlegierungen der alten und ältesten Völker (Erlangen, 1869), pp. 60–61, 64–65.
|
| 70 |
Stohman, F. and Kerl, K., Encyklopädisches Handbuch der Technischen Chemie (Braunschweig, 1888–1905), IV, pp. 2013–2014.
|
| 71 |
Op.cit., pp. 52–55.
|
| 72 |
Hofmann, K. B., Numismatische Zeitschrift, XVI (1884), p. 10.
|
| 73 |
Helm, O., Zeitschrift für Ethnologie, XXVII (1895), pp. 19–20.
|
| 74 |
Grueber, H. A., Numismatic Chronicle, Ser. 4, IV (1904), p. 244.
|
| 75 |
Grant, M., From Imperium to Auctoritas (Cambridge, 1946), p. 457.
|
| 76 |
Mattingly, H., B.M.C. Coins of the Roman Empire (London, 1923), I, p. 32.
|
| 77 |
Forrer, R., Zeitschrift für Ethnologie, XLI (1909), pp. 458–462.
|
| 78 |
Forrer, R., Keltische Numismatik der Rhein- und Donaülande (Straβburg, 1908).
|
| 79 |
Mattingly, H., B.M.C. Coins of the Roman Empire (London, 1923-), I, p. lvii; III, pp. xxi-xxii; IV, pp. xvi-xvii.
|
| 80 |
With the exception of the analyses listed by Hammer, J., Der Feingehalt der griechischen und römischen Münzen (Diss. Tübingen, 1906). However, those listed by Hammer are merely compiled from the publications of other writers, chiefly
Bibra.
|
| 81 |
Grant, M., From Imperium to Auctoritas (Cambridge, 1946), p. 493.
|
The unsatisfactory state of our knowledge of the composition of orichalcum coins based on previous analyses has been remedied to a considerable extent by the careful analyses of twenty-five representative and adequately identified coins of this class executed in the author's laboratory by various students within the past twenty-five years. Ten of these coins were duplicates from the collections of the American Numismatic Society and were kindly supplied for analysis by Dr. George C. Miles, Chief Curator. The others were purchased by the author from various dealers at various times. Table XVI contains a descriptive list of the coins analyzed. The names of the analysts, to whom the author is much indebted for their painstaking work, are given at the end of the descriptions in order of the extent of their contributions.
TABLE XVI
list of coins analyzed
1. Emperor = Augustus
Date = 23 b.c. (Mattingly) or 19–16 b.c. (Grant)
Weight = 12.4 grams
Denomination = Dupondius
Size = 26–27 mm. Condition = Fine
Obv.: AVGVSTVS in three lines
TRIBVNIC in oak-wreath.
POTEST
Rev.: L · SVRDINVS · IIIVIR · A · A · A · F · F · around S C.
Ref. BMC, Vol. I, p. 30, No. 141, pi. 19, No. 2.
Analyst: W. H. Deebel.
2. Emperor = Augustus
Date = 22 b.c. (Mattingly) or 19–16 b.c. (Grant)
Weight = 20.6 grams
Denomination = Sestertius
Size = 33–34 mm. Condition = Good
Obv.: Oak-wreath between laurel branches
OB above wreath
CIVIS within wreath
SERVATOS below wreath
Rev.: C · ASINIVS · C · F · GALLVS · III · VIR · A · A · A · F · F ·
S C large in center
Ref. BMC, Vol. I, p. 32, No. 157.
Analysts: M. C. Suarez, D. Loyer, D. Perez and J. Fratz.
3. Emperor = Tiberius
Date = a.d. 22–23
Weight = 11.8 grams
Denomination = Dupondius
Condition = Good, but patinated and worn
Size = 28–29 mm.
Obv.: Tl · CAESAR · DIVI · AVG · F · AVG · P · M · TR · POT · XXIIII starting at top. S C large in center.
Rev.: Bust of Livia as Justitia(P), draped r., wearing stephane ornamented with floral ornaments; her hair fastened in a knot at the back. IVSTITIA below bust, outwardly.
Ref. BMC, Vol. I, p. 131, No. 79 or 80.
Analysts: M. C. Suarez, T. Hutt, D. Loyer, D. Perez and J. Fratz.
4. Emperor = Caligula
Date = a.d. 37–41
Weight = 12.2 grams
Denomination = Dupondius
Condition = Good
Size = 28–29 mm.
Obv.: Head of Augustus, radiate, l.
DIVVS AVGVSTVS in arc above.
SCI. and r. in field.
Rev.: Augustus, laureate, togate, seated l. on curule chair, holding branch in r. hand and resting l. hand against side.
CONSENSV · SENAT · ET · EQ · ORDIN · P · Q · R starting low l.
Ref. BMC, Vol. I, p. 160, Nos. 88–91.
Analysts: M. C. Suarez, T. Hutt, D. Perez, D. Loyer and J. Fratz.
5. Emperor = Caligula
Date = a.d. 37–38
Weight = 25.8 grams
Denomination = Sestertius
Condition = Fair, but holed and corroded
Size = 35–36 mm.
Obv.: Head of Caligula, laureate, l.
C · CAESAR · AVG · GERMANICVS · PON · M · TR · POT · starting low l.
Rev. : [S · P · Q · R]
P P in four lines in an oak-wreath.
OB CIVES
SERVATOS
Ref. BMC, Vol. I, p. 152, No. 38 or 39.
Analysts: M. C. Suarez and T. Hutt.
6. Emperor = Caligula
Date = a.d. 39–40
Weight = 25.4 grams
Denomination = Sestertius
Condition = Poor (much worn and corroded)
Size = 35 mm.
Obv.: Head of Caligula, laureate, l.
[C · CAESAR · DLV]I · AVG · PRON · [AVG · P · M · TR · P.]
III [P P]
Rev.: Caligula, bare-headed, togate, standing l. on a low platform on r., extending r. hand in gesture of address; behind him on platform a low chair. In front of him stand five soldiers r., all helmeted, holding shields and parazonia; the foremost soldier stands alone, the other four in two files, and each of these carries an aquila. Inscription illegible.
Ref. BMC, Vol. I, p. 156, No. *, pi. 28, No. 7.
Analysts: B. Becker and N. Lovegren.
7. Emperor = Caligula
Date = a.d. 39–40
Weight = 23.8 grams
Denomination = Sestertius
Condition = Fair
Size = 34–35 mm.
Obv.: Head of Caligula, laureate, l.
C · CAESAR · DIVI · AVG · PRON · AVG · P · M · TR · P · III ·
P.P.
Rev.: S · P · Q · R
P · P in an oak-wreath.
OB · CIVES
SERVATOS
Ref. BMC, Vol. I, p. 156, No. 58, pi. 28, No. 8.
Analysts: W. H. Deebel, D. Loyer, D. Perez and J. Fratz.
8. Emperor = Claudius
Date = a.d. 41
Weight = 22.7 grams
Denomination = Sestertius
Condition = Fair
Size = 34–35 mm.
Obv.: Head of Claudius, laureate, r.
Tl · CLAVDIVS · CAESAR · AVG · P · M · TRP · IMP ·
Rev.: EX · S · C
OB in an oak-wreath.
CIVES
SERVATOS
Ref. BMC Vol. I, p. 181, No. 115, pi. 34, No. 9.
Analysts: W. H. Deebel, T. Hutt, D. Perez, D. Loyer and J. Fratz.
9. Emperor = Claudius
Date = a.d. 41
Weight = 12.4 grams
Denomination = Dupondius
Condition = Fair, but corroded
Size = 27–29 mm.
Obv.: Bust of Antonia, draped r., head bare, hair fastened in long plait at back
ANTONIA A[VGVSTA] off flan. l. up, r. down.
Rev.: Claudius, togate, veiled, standing l., holding simpulum in r. hand and resting l. hand on his side.
Tl · [CLAVDIVS · CAESAR · AVG ·] P · M · TR · P · IMP · partly off flan, starting low left.
SCI. and r., low in field.
Ref. BMC, Vol. I, p. 188, No. 166.
Analysts: M. C. Suarez, T. Hutt, D. Loyer, D. Perez and J. Fratz.
10. Emperor = Nero
Date = a.d. 64–66
Weight = 12.4 grams
Denomination = Dupondius
Condition = Good, but corroded
Size = 27–29 mm.
Obv.: Head of Nero, radiate, l.
NERO CLAVDIVS CAESAR AUG GER PM TRP IMP PP.
Rev.: View of Temple of Janus, showing one front and one side. Left-hand side shown.
PACE P · R · TERRA MARIQ · PARTA IANVM CLVSIT SCI. and r. in field.
Ref. BMC, Vol. I, p. 238, No. 200.
Analyst: M. C. Suarez.
11. Emperor = Nero
Date = a.d. 64–66
Weight = 17.9 grams
Mint = Rome
Denomination = Dupondius
Condition = Good
Size = 29 mm.
Obv.: Head of Nero, radiate, r.
NEROCLAVDCAESARGERPMTRPIMPPP
Rev.: Victory, draped, striding l., holding wreath in r. hand and palm in l.
VICTORIA AVGVSTI l. up, r. down.
SCI. and r. in field. II in ex.
Ref. BMC, Vol. I, pp. 241–42, Nos. 214–16 (Type E).
Analysts: R. L. Wicinski, T. Hutt, D. Loyer, D. Perez and J. Fratz.
12. Emperor = Nero
Date = a.d. 66–67
Weight = 12.1 grams
Mint = Lugdunum
Denomination = Dupondius
Condition = Very good
Size = 30 mm.
Obv.: Head of Nero, laureate, r.
IMPNEROCAESARAVGPMAXTRPPP
Rev.: Victory, draped, advancing l., holding wreath in r. hand and palm in l.
VICTORIA AVGVSTI l. up, r. down.
SCI. and r. in field.
Ref. BMC, Vol. I, pp. 269–70, Nos. 353–55 (Type D).
Analyst: R. L. Wicinski.
13. Emperor = Nerva
Date = a.d. 97
Weight = 11.5 grams
Denomination = Dupondius
Condition = Good
Size = 26 mm.
Obv.: Head of Nerva, radiate, r.
IMPNERVACAESAVG PMTRPCOSIIIPP
Rev.: Fortuna, draped, standing l., holding rudder on ground in r. hand and cornu copiae in l.
FORTVN[A AVGVJST
SCI. and r., in field.
Ref. BMC, Vol. Ill, p. 22, Nos. 123–25.
Analysts: M. C. Suarez and T. Hutt.
14. Emperor = Nerva
Date = a.d. 96–98
Weight = 12.7 grams
Denomination = Dupondius
Condition = Good
Size = 27 mm.
Obv.: Head of Divus Augustus, radiate, r.
[DIVVS] AVGVSTVS
Rev.: Rudder upright, over globe.
IMP NERVA CAES AVG REST
S C in exergue.
Ref. BMC, Vol. Ill, p. 29, No. 154, pi. 8, No. 2.
Analysts: A. Randall and N. Lovegren.
15. Emperor = Trajan
Date = a.d. 98–100
Weight = 10.0 grams
Denomination = Dupondius
Condition = Poor (corroded and worn)
Size = 26–27 mm.
Obv.: Head of Trajan, radiate, r.
IMP CAES NERVA TRAIAN AVG [.......]
Rev.: Woman (Justitia?) seated l. on a throne with cornua copiae as arms, holding vertical sceptre in r. hand, l. hand on lap, fold of drapery falling over lap.
[.......]COS[...]
[..] in exergue.
Ref. BMC, Vol. Ill, p. 149, No. 719 or p. 152, No. 734.
Analyst: M. C. Suarez.
16. Emperor = Trajan
Date = a.d. 98–100
Weight = 11.1 grams
Denomination = Dupondius
Condition = Fair
Size = 26–28 mm.
Obv.: Head of Trajan, radiate, r.
IMP CAES NERVA TRAIAN AVG GERM PM
Rev.: Woman (Justitia?) seated l. on a throne with cornua copiae as arms, holding vertical sceptre in r. hand, l. hand on lap, fold of drapery falling over lap.
TR POT COS[...]
S C in exergue.
Ref. BMC, Vol. III, p. 149, No. 719 or p. 152, No. 734.
Analysts: M. C. Suarez and T. Hutt.
17. Emperor = Trajan
Date = a.d. 116–117
Denomination = Sestertius
Size = 34 mm.
Condition = Poor (most of right half broken off, remainder bent).
Obv.: Bust of Trajan, draped, laureate, r.
IMP CAES NER TRIANO OPTIMO AVG GER DA [........].
Rev.: Providentia draped.
PROVIDENTIA [...........].
Ref. BMC, Vol. Ill, p. 222, No. 1041.
Analysts: M. C. Suarez, T. Hutt, D. Perez, D. Loyer and J. Fratz.
18. Emperor = Trajan
Date = a.d. 112–114(?)
Weight = 10.2 grams
Denomination = Dupondius
Condition = Fair
Size = 26 mm.
Obv.: Bust of Trajan, radiate, draped, r.
IMP CAES NERVAE TRAIANO
AVG GER DAC PM TRP COS VI PP
Rev.: Column of Trajan.
S C (large) l. and r. in field.
Legend illegible.
Ref. BMC, Vol. Ill, p. 210, No. 994, pi. 39, No. 4.
Analysts: P. E. Machemer and N. Lovegren.
19. Emperor = Antoninus Pius
Date = a.d. 141 or shortly after.
Weight = 25.4 grams.
Denomination = Sestertius
Condition = Fair
Size = 31–32 mm.
Obv.: Bust of Faustina, draped, r.
DIVA AVGVSTA FAVSTINA
Rev.: Pietas standing l., dropping incense on candelabrum and holding box of perfumes.
PIETAS AVG Infield, SC
Ref. BMC, Vol. IV, p. 233, Nos. 1447–1448.
Analysts: W. H. Deebel, D. Loyer, D. Perez and J. Fratz.
20. Emperor = Antoninus Pius
Date = a.d. 141 or shortly after.
Weight = 11.2 grams.
Denomination = Dupondius
Condition = Fair/Poor.
Size = 24–26 mm.
Obv.: Bust of Faustina, draped, r.
DIVA AVGVSTA FAVSTINA
Rev.: Pietas standing l., dropping incense on candelabrum and holding box of perfumes.
PIETAS AVG In field, S C
Ref. BMC, Vol. IV, p. 236, No. 1468.
Analyst: W. H. Deebel.
21. Emperor = Antoninus Pius
Date = a.d. 154–155
Weight = 12.6 grams.
Denomination = Dupondius
Condition = Fair/Poor
Size = 25–26 mm.
Obv.: Head of Antoninus Pius, radiate, r.
ANTONINVS AVG PIVS PP TRP XVIII
Rev.: Libertas standing r., holding pileus and sceptre.
LIBERTAS COS 1111 In field, S C
Ref. BMC, Vol. IV, p. 330, No. 1969; pi. 48, No. 17 (reverse only).
Analysts: W. H. Deebel, T. Hutt, D. Loyer, D. Perez and J. Fratz.
22. Emperor = Antoninus Pius
Date = a.d. 154–155
Weight = 13.9 grams
Denomination = Dupondius
Condition = Fair/Poor
Size = 25–26 mm.
Obv.: Head of Antoninus Pius, radiate, r.
ANTONINVS AVG PIVS PP TRP XVIII
Rev.: Libertas standing r., holding pileus and extending r. hand.
LIBERTAS COS IIII In field, S C
Ref. BMC, Vol. IV, p. 330, No. 1967.
Analysts: W. H. Deebel, D. Perez, D. Loyer and J. Fratz.
23. Emperor = Marcus Aurelius
Date = a.d. 162–163
Weight = 22.6 grams.
Denomination = Sestertius
Condition = Fair/Poor
Size = 30–31 mm.
Obv.: Head of Marcus Aurelius, laureate, r.
IMP CAES M AVREL ANTONINVS AVG PM
Rev.: Salus standing l., feeding snake twined around altar.
SALVTI AVGVSTOR TRP XVII COS III
In field, S C
Ref. BMC, Vol. IV, p. 550, No. 1037.
Analysts: W. H. Deebel, D. Perez, D. Loyer and J. Fratz.
24. Emperor = Marcus Aurelius
Date = a.d. 161–162
Weight = 12.0 grams.
Denomination = Dupondius
Condition = Poor
Size = 24–26 mm.
Obv.: Head of Marcus Aurelius, radiate, r.
IMP CAES M AVREL ANTONINVS AVG PM
Rev.: Marcus Aurelius and Lucius Verus standing with clasped hands. Aurelius holds a scroll.
CONCORD AVGVSTOR TRP XVI COS III
In field, S C
Ref. BMC, Vol. IV, p. 547, No. 1016, pi. 75, No. 1.
Analysts: W. H. Deebel, T. Hutt, D. Loyer, D. Perez and J. Fratz.
25. Emperor = Commodus
Date = a.d. 179
Weight = 12.9 grams.
Denomination = Dupondius
Condition = Good/Fair
Size = 24–26 mm.
Obv.: Head of Commodus, radiate, r.
L AVREL COMMODVS AVG TRP III
Rev.: Victory advancing l., holding wreath and palm
IMP III COS II PP In field, SC
Ref. BMC, Vol. IV, p. 680, No. 1708
Analysts: W. H. Deebel, T. Hutt, D. Loyer, D. Perez and J. Fratz.
Six of the coins (Nos. 2, 7, 8, 19, 22 and 23) were examined by X-ray fluorescence analysis in the hope that a method could be used that would not damage the coins. This hope was not realized since the examination of different areas of the untreated surfaces of the coins yielded very discordant results for both the major and minor components of the alloys. This is understandable in view of the visually heterogeneous character of most of these surfaces. Reproducible quantitative results by this method of analysis can be obtained only if the X-ray beam is directed on plane and smooth metal surfaces that have been carefully prepared so as to resemble very closely the surfaces of the reference standards. Even when one surface of each of the coins was ground smooth and polished, the results were inferior in precision, accuracy, and completeness to those obtainable by chemical methods of analysis. The author is indebted to Mr. J. Manchester and the late Professor W. MacNevin for assistance in the attempts to apply X-ray fluorescence analysis to the determination of the composition of orichalcum coins.
In sampling the coins for chemical analysis, the usual procedure was to remove with a clean file all the corroded surface metal and a sufficient layer of metal below the surface to obtain a blank of metal that was free from visible corrosion and which was presumably of the same composition as the original metal. Sometimes cracks had to be cleaned out with a file or saw, and local pits of corroded metal with a drill. Sectors of clean metal that weighed about a gram were then cut from these blanks with a clean saw. At least two sectors from each blank were analyzed for all the usual components. Often additional sectors were taken for the purpose of checking determinations when doubt arose as to their correctness, or for the purpose of making special tests or determinations. For exact analysis this method of destructive sampling is much to be preferred to the removal of small samples from the edge or faces of a coin, for such small samples are very likely not to be truly representative of the composition of the original alloy, especially with orichalcum coins. Indeed, by reason of selective corrosion, the surface metal of some orichalcum coins has been found to be nearly free from zinc, so that the analysis of small samples taken from the surface of such coins would yield very deceptive results.
The outline of the analytical procedure which follows is intended only to indicate the general scheme that was used so that its validity may be judged. Most of the necessary manipulative details have been omitted since these are to be found in the standard works on chemical analysis.
Accurately weighed samples of about a gram were treated with nitric acid for the separation of the gold and tin from the other metals. The ignited and weighed residue from the nitric acid treatment was extracted with cold, dilute aqua regia to dissolve the gold, and the resultant solution was treated with either ferrous sulfate solution or oxalic acid solution to precipitate the gold. Only a trace of gold was usually found, but when a weighable amount was present this was collected on filter paper, ignited, and weighed. By subtracting the weight of gold from the weight of the residue, the weight of stannic oxide was obtained, from which the weight of the tin was calculated. When the light color of the nitric acid residue showed that gold was absent, the treatment with aqua regia was omitted, and the weight of the tin was calculated directly from the weight of the residue.
The filtrate from the separation of the tin, or both tin and gold, was treated with hydrochloric acid solution to precipitate silver as the chloride. The silver chloride was collected in a weighed filter crucible, and, after drying and weighing, the weight of the silver chloride was found, from which the weight of the silver was calculated. The filtrate from the separation of the silver was treated with sulfuric acid, and the solution was evaporated until fumes of sulfur trioxide appeared. After cooling, the residue was treated with water, and the lead sulfate was collected in a weighed filter crucible, dried and weighed. Copper was determined by electrolysis in the filtrate from the separation of the lead, and from the weight of the small amount of lead dioxide deposited on the anode, and the weight of the lead sulfate, the total lead content was calculated. The filtrate from the separation of the copper and residual lead was evaporated to a small volume and treated with ammonium hydroxide solution to precipitate the iron. The precipitate was collected on filter paper and ignited to ferric oxide in a weighed crucible, the amount of iron being found from the weight of this oxide. In the filtrate from the separation of the iron, nickel was precipitated with dimethylglyoxime solution. The precipitate was col- lected in a weighed filter crucible, dried and weighed, and the amount of nickel was calculated from the weight of this precipitate. After treatment of the filtrate from the separation of the nickel with nitric acid to destroy organic matter and remove the excess of ammonium salts, the resultant solution was diluted, neutralized, and treated with ammonium phosphate solution to precipitate the zinc. The precipitate was collected in a weighed filter crucible and dried or ignited to obtain a residue of zinc phosphate suitable for weighing. The amount of zinc was calculated from the weight of the zinc phosphate.
Most of the coins were examined for the presence of arsenic and sulfur, separate samples being used for this purpose. Some of the same samples used for arsenic were tested for the presence of antimony. Arsenic was determined by first treating a weighed sample with concentrated nitric acid until decomposed. Concentrated sulfuric acid was then added and the solution was evaporated until all the nitric acid and nitrates were removed. This solution was diluted with water and transfered to a distilling flask provided with a dropping funnel and connected with a condenser and a receiving flask provided with a series of traps. Solid ferrous sulfate or a solution of hypophosphorous acid was added to the solution in the distilling flask, the dropping funnel was filled with concentrated hydrochloric acid, and the proper volumes of water placed in the receiver and traps. The solution of the sample was then distilled with the frequent addition of acid from the dropping funnel. When most of the solution had distilled over and the temperature of the vapor had reached 108° C the distillation was stopped. The liquid in the receiver and traps was flushed into a flask, and the arsenic was precipitated with a stream of hydrogen sulfide gas. The precipitate of arsenious sulfide, more or less contaminated with sulfur, was collected in a weighed filter crucible, dried and weighed. After treatment of the dried precipitate with ammonium hydroxide solution to dissolve out the sulfide, the crucible with its residual sulfur was again dried and weighed to find the weight of pure arsenious sulfide by difference. The amount of arsenic was calculated from this weight. Concentrated phosphoric acid was then added to the solution remaining in the distillation flask, and the process of distillation was repeated at a higher temperature. The liquid in the receiver and traps was treated with hydrogen sulfide gas for the detection of antimony, but it was not found by this test in any of the samples. Sulfur was determined by first treating a weighed sample with concentrated nitric acid, evaporating to small volume, diluting with water, and filtering to remove any residue. The filtrate was evaporated nearly to dryness twice with concentrated hydrochloric acid and diluted with water. Any precipitate of silver chloride was removed by filtration, and the clear solution was treated with barium chloride solution to precipitate sulfur as barium sulfate, which was filtered off in a weighed filter crucible, washed, dried and weighed. If the dried precipitate was not entirely white, it was dissolved out of the crucible by treatment with successive small volumes of concentrated sulfuric acid, and the resultant solution was allowed to flow into water to reprecipitate the barium sulfate. The purified precipitate was collected in a weighed filter crucible, washed, dried and weighed as before. The amount of sulfur was calculated from the weight of either the original or the purified precipitate. 82
Samples from eight of the coins (Nos. 2, 4, 5, 7, 8, 19, 23 and 24) were examined spectrographically for very small proportions of minor components that may have escaped detection by the above procedure, and for elements which might not be detected at all by this procedure. The samples, each of which weighed 10 milligrams, were examined both by direct vaporization in the carbon arc and by vaporization of their solutions in aqua regia first absorbed in the electrodes. An alternating current arc was used, and the spectral range was from 2130 to 4350 Angstrom units. For the estimation of any elements found qualitatively, the semi-quantitative method of Harvey 83 was used. The author is indebted to Mrs. Nulifer I. Woods, a graduate student, for all of the spectrographic results.
The results of the analyses are shown in Tables XVII and XVIII. Each result in Table XVII, except for the totals of the minor components and the summations, is the average of two or more separate determinations which nearly always agreed closely with one another. With a few exceptions each result shown in Table XVIII is also the average of at least two determinations. The minus signs in this table indicate that determinations of the given elements were not made. Most of the summations are sufficiently close to 100.00% to indicate that all the elements present in significant proportion were determined. The totals for Nos. 10 and 12 may be low because of the presence of undetermined arsenic and sulfur, though it appears more likely that the deficiencies are due to the presence of undetermined oxygen, which was undoubtedly present to some extent in the form of metal oxides in all the coins. The high summations (i.e., over 100.00%) arise from positive experimental errors in the determinations and
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Other Components % | Total % |
| 1 | 77.36 | 21.88 | 0.17 | 0.16 | 0.41 | 99.98 |
| 2 | 76.70 | 22.02 | 0.27 | 0.37 | 0.57 | 99.93 |
| 3 | 76.86 | 22.85 | 0.03 | 0.04 | 0.29 | 100.07 |
| 4 | 72.63 | 26.71 | 0.02 | 0.16 | 0.52 | 100.04 |
| 5 | 77.52 | 22.20 | 0.04 | 0.01 | 0.32 | 100.09 |
| 6 | 78.19 | 21.11 | 0.12 | 0.04 | 0.39 | 99.85 |
| 7 | 81.03 | 18.55 | none | 0.05 | 0.25 | 99.88 |
| 8 | 75.91 | 23.20 | 0.09 | 0.12 | 0.74 | 100.05 |
| 9 | 77.59 | 21.11 | 0.10 | 0.83 | 0.54 | 100.17 |
| 10 | 83.16 | 15.95 | 0.01 | 0.13 | 0.49 | 99.74 |
| 11 | 77.27 | 22.46 | none | 0.15 | 0.29 | 100.17 |
| 12 | 78.24 | 20.98 | none | 0.14 | 0.29 | 99.65 |
| 13 | 83.60 | 14.82 | 0.70 | 0.54 | 0.52 | 100.18 |
| 14 | 84.69 | 13.59 | 0.57 | 0.49 | 0.45 | 99.79 |
| 15 | 81.21 | 17.76 | 0.02 | 0.37 | 0.49 | 99.85 |
| 16 | 83.48 | 16.01 | 0.02 | 0.10 | 0.33 | 99.94 |
| 17 | 82.08 | 14.10 | 2.05 | 1.29 | 0.61 | 100.13 |
| 18 | 76.83 | 14.45 | 4.34 | 4.33 | 0.19 | 100.14 |
| 19 | 86.79 | 12.33 | none | 0.13 | 0.84 | 100.09 |
| 20 | 86.28 | 12.71 | 0.06 | 0.32 | 0.59 | 99.96 |
| 21 | 86.51 | 11.14 | 1.69 | 0.11 | 0.73 | 100.18 |
| 22 | 89.29 | 9.38 | 0.16 | 0.35 | 0.97 | 100.15 |
| 23 | 87.86 | 9.06 | 2.03 | 0.23 | 0.87 | 100.05 |
| 24 | 88.96 | 7.87 | 2.33 | 0.18 | 0.88 | 100.22 |
| 25 | 86.85 | 6.43 | 1.64 | 4.28 | 0.86 | 100.06 |
| Coin No. | Iron % | Nickel % | Silver % | Gold % | Arsenic % | Sulfur % | Total % |
| 1 | 0.38 | none | none | none | — | 0.03 | 0.41 |
| 2 | 0.49 | 0.04 | 0.04 | none | trace | none | 0.57 |
| 3 | 0.26 | 0.03 | none | none | none | none | 0.29 |
| 4 | 0.44 | 0.01 | 0.03 | none | 0.04 | — | 0.52 |
| 5 | 0.27 | 0.03 | trace | none | 0.02 | none | 0.32 |
| 6 | 0.39 | none | none | none | none | none | 0.39 |
| 7 | 0.22 | 0.03 | trace | none | none | none | 0.25 |
| 8 | 0.67 | 0.02 | 0.01 | trace | 0.04 | none | 0.74 |
| 9 | 0.47 | 0.02 | 0.03 | trace | 0.02 | none | 0.54 |
| 10 | 0.40 | 0.03 | 0.06 | none | — | — | 0.49 |
| 11 | 0.16 | none | 0.08 | — | 0.05 | none | 0.29 |
| 12 | 0.24 | trace | 0.05 | — | — | — | 0.29 |
| 13 | 0.46 | 0.03 | none | trace | 0.03 | — | 0.52 |
| 14 | 0.38 | 0.05 | none | none | 0.02 | none | 0.45 |
| 15 | 0.30 | 0.04 | 0.08 | none | 0.07 | — | 0.49 |
| 16 | 0.26 | 0.04 | 0.03 | trace | — | — | 0.33 |
| 17 | 0.37 | 0.04 | 0.09 | none | 0.03 | 0.08 | 0.61 |
| 18 | 0.12 | 0.04 | 0.03 | — | none | none | 0.19 |
| 19 | 0.58 | 0.03 | 0.01 | 0.04 | 0.06 | 0.12 | 0.84 |
| 20 | 0.51 | 0.02 | 0.06 | trace | — | — | 0.59 |
| 21 | 0.33 | 0.03 | 0.10 | trace | 0.10 | 0.17 | 0.73 |
| 22 | 0.55 | 0.04 | 0.05 | trace | 0.12 | 0.21 | 0.97 |
| 23 | 0.33 | 0.03 | 0.10 | none | 0.08 | 0.28 | 0.87 |
| 24 | 0.31 | 0.04 | 0.05 | trace | 0.21 | 0.17 | 0.88 |
| 25 | 0.28 | 0.06 | 0.07 | trace | 0.13 | 0.32 | 0.86 |
Notes
Many of the results for copper and zinc shown in Table XVII differ decidedly from those found by previous analyses, the proportions of copper being generally lower and the proportions of zinc generally higher for the coins of any given emperor. For example, the average figures for the copper and zinc content of the coins of Augustus in Table XVII are 77.03% and 21.55%, respectively, whereas the corresponding average figures for all previous analyses are 82.3% and 17.0%. In view of the complexity of the coinage of Augustus the differences between these sets of figures might be ascribed entirely to the analysis of coins of widely different class or type by the several analysts, but similar differences, usually less in degree, exist between the results of these new analyses and the results of previous analyses for the coins of most of the other emperors. For example, the average proportions of copper and zinc in the four coins of Caligula of Table XVII are77.34% and 22.14%,respectively, whereas the corresponding figures for the two previous analyses are 79.8% and 20.2%. In Table XIX are shown for comparison the average percentages of copper and zinc, according to the best previous analyses and these new analyses, in orichalcum coins of the first century a.d. that were issued during the reigns of Tiberius to Nerva, inclusive; a range of time of issue here designated as Period I. It will be seen that the average results for the copper content differ by more than 4% and that the average results for the zinc content differ by almost 5%. The differences between the results of previous analyses and the results of these new analyses for the copper and zinc content of coins issued in the second century a.d. are generally smaller, as is indicated by the comparison of the average results shown in Table XIX for orichalcum coins issued during the reigns of the emperors from Trajan to Commodus inclusive; a range of time of issue here designated as Period II. It will be seen that the copper content differs by only a little more than 1% and the zinc content by a little less than 2%. Nevertheless, for the coins of this period also, the copper content is definitely lower and the zinc content definitely higher according to these new analyses. Though intrinsic differences undoubtedly existed between the composition of coins of given emperors taken for the earlier analyses and these new analyses, this does not seem a sufficient cause for the general lack of agreement between the earlier results and the new results for the percentages of copper and zinc, especially since this lack of agreement is in the same direction over the entire time of issue. Nor does it seem likely that this lack of agreement can be ascribed to differences in the methods of analysis or skill of the analysts. The most likely cause appears to be differences in the care taken in the preparation of samples for analysis. Most of the previous analysts say nothing about their method of sampling, from which it would seem likely that little attention was paid to this important matter. If they had taken their samples merely by cutting pieces from uncleaned coins, or if they had failed to remove much of the corroded metal from the pieces they analyzed, this would account for the observed lack of agreement, for the corrosion products of orichalcum coins contain a higher proportion of copper and a lower proportion of zinc than the uncorroded metal. Bibra, who analyzed more orichalcum coins than any other single investigator, does indeed describe his method of sampling, but it is evident from his description that his method was faulty. 84 He customarily removed the corrosion products from the metal objects he analyzed by means of concentrated ammonium hydroxide solution or concentrated nitric acid. This chemical cleaning method when applied to orichalcum coins must have tended to some extent to dissolve out the zinc preferentially from the surface, and consequently he obtained slightly high results for the copper content and slightly low results for the zinc content of the samples he analyzed. By reason of a better method of sampling, if for no other reason, the new results shown in Table XVII for the copper and zinc content of orichalcum coins are closer to the truth than previous results for these principal components.
| Group of Analyses | Number of Analyses | Period | Copper % | Zinc % |
| Previous | 9 | 1 | 83.10 | 15.34 |
| New | 12 | 1 | 78.89 | 20.29 |
| Previous | 25 | 11 | 86.22 | 10.05 |
| New | 11 | 11 | 85.10 | 11.93 |
The results in Table XVII for the tin and lead content of orichalcum coins of the same emperor or successive emperors will be seen to vary in an erratic way, the only distinct trend being that the higher percentages of both metals usually occur in the coins of the later emperors. In these respects the result of these new analyses agree with the results of previous analyses, though there are differences in the average percentages of tin and lead as shown by the figures in Table XX. The average of previous results for coins of Period I is based only on the analyses published by Bibra and by Helm (Tables X and XI) since other previous analysts either report no results for the tin or lead content of the coins they analyzed or report results of very questionable accuracy. It will be seen that the average of previous results for the tin content of coins of this period is considerably higher than the average of the new results, and that the average of the previous results for the lead content is slightly lower. If one apparently atypical set of results obtained by Bibra (Table X, No. 9) had been included in computing the average tin content from previous analyses, this average would have been 0.94% as compared to the 0.14% found by the new analyses, and the average lead content would have been 0.24%, almost identical with the average of the new results for Period I. For the coins of Period II the average tin content computed from the results of previous analyses is also much higher than the average of the results of these new analyses, and the average lead content is distinctly lower. These differences between the averages of the previous results and the new results for the tin content of orichalcum coins may also be explained to a large extent from the differences in care exercised in sampling coins for analysis. The inclusion of some corrosion products in samples taken for analysis, which appears to have been the practice of some of the previous analysts, would yield high results for tin, not only because tin as the insoluble oxide would be present in higher proportion in these corrosion products than in the alloy itself, but because other insoluble matter such as clay and sand trapped in the corrosion products would remain with the tin oxide formed on treating the alloy with nitric acid for analysis and would be weighed along with this oxide. The lower average proportions of lead found by previous analysts is probably due only in part to differences in care exercised in sampling. To some extent this lower proportion may be ascribed to an error in the method of determining the lead. Apparently all the analysts separated and determined the lead as sulfate in aqueous solution but did not correct for the lead sulfate remaining in solution. In the procedure used for these new analyses the lead remaining in solution was recovered and determined electro-lytically, so that all the lead was determined.
| Group of Analyses | Number of Analyses | Period | Tin % | Lead % |
| Previous | 5 | 1 | 0.49 | 0.15 |
| New | 12 | 1 | 0.14 | 0.23 |
| Previous | 21 | 11 | 2.20 | 0.65 |
| New | 11 | 11 | 1.30 | 1.06 |
As will be seen from Table XVIII, iron was found to be present in small proportions in all the orichalcum coins that were analyzed. Genth, Bibra, and Helm (Tables IX, X, and XI, respectively) also report iron to be an invariable component of such coins. Other previous analysts either do not report iron as being present, or report it as being present only in some of the coins they analyzed. Apparently they did not attempt its determination in the coins for which no results are reported. As shown in Table XVIII, the proportions of iron found by these new analyses range from 0.12% to 0.67%. The proportions found by Genth and by Helm all fall within this range, but nearly a third of the results reported by Bibra fall outside of it. A comparison of the distribution of the results reported by Bibra with the distribution of the results of the new analyses is given in Table XXI. Since iron appears to be a purely accidental impurity in orichalcum, the approximately equal distribution of the results of a series of determinations around a central point, as found by these new analyses, seems inherently more likely. Moreover, a comparison of all the known results for the iron content of the orichalcum coins of a given emperor, as shown by the example in Table XXII, indicates that Bibra's results for iron are not accurate. Further comparisons of the results obtained by Bibra with the results of the new analyses are shown in
| Number in Range | ||
| Iron Content % | As Reported by Bibra | According to New Analyses |
| 0.01–0.10 | 1 | 0 |
| 0.11–0.20 | 4 | 2 |
| 0.21–0.30 | 0 | 7 |
| 0.31–0.40 | 6 | 8 |
| 0.41–0.50 | 1 | 4 |
| 0.51–0.60 | 2 | 3 |
| 0.61–0.70 | 1 | 1 |
| 0.71–0.80 | 1 | 0 |
| 0.81–0.90 | 0 | 0 |
| 0.91–1.00 | 2 | 0 |
| 1.01–1.10 | 3 | 0 |
| 1.11–1.20 | 1 | 0 |
| 1.21–1.30 | 1 | 0 |
| –– | –– | |
| Totals | 23 | 25 |
| Analyst | Iron % |
| Genth | 0.29 |
| Helm | 0.20 |
| Helm | 0.15 |
| Av. = 0.21 | |
| Bibra | 1.00 |
| Bibra | 0.39 |
| Av. =070 | |
| Suarez | 0.37 |
| Suarez | 0.30 |
| Suarez | 0.26 |
| Machemer | 0.12 |
| Av. = 0.26 |
| Source of Data | Metal | Maximum % | Minimum % | Average % |
| Bibra's Analyses | Iron | 1.30 | 0.01 | 0.57 |
| New Analyses | Iron | 0.67 | 0.12 | 0.37 |
| Bibra's Analyses | Nickel | 0.50 | trace | 0.22 |
| New Analyses | Nickel | 0.06 | none | 0.03 |
| Source of Data | Number of Analyses | Period | Iron % | Nickel % |
| Bibra's Analyses | 5 | I | 0.31 | 0.17 |
| New Analyses | 12 | I | 0.36 | 0.02 |
| Bibra's Analyses | 16 | II | 0.64 | 0.25 |
| New Analyses | 11 | II | 0.36 | 0.04 |
As shown in Table XVIII, nickel was found in small and not very different proportions in a large majority of the orichalcum coins that were analyzed, actually in twenty-one out of the twenty-five. Proportions of nickel too small to detect by the method that was used may have been present in the few coins in which none was found. Bibra and Helm (Tables X and XI, respectively) are the only previous analysts who reported the presence of nickel in orichalcum coins. Bibra reported that nickel was present in all the coins he analyzed, though in determinable proportions in only eighteen out of the twenty-three. Helm reported determinable proportions in two out of four. However, the proportions of nickel reported by Bibra and by Helm are much higher than those found by these new analyses. Summarized comparisons of the nickel determinations reported by Bibra and those now reported are shown in Tables XXIII and XXIV. The generally high results for nickel obtained by Bibra are undoubtedly due to his use of an inaccurate method for its determination. The inaccuracy of Bibra's method has been demonstrated by Ibbotson, 85 who, by means of a series of trial analyses, found that this method, though trustworthy from a qualitative standpoint, is liable under certain conditions to yield high quantitative results.
Nickel, like iron, is an accidental impurity in orichalcum, but with the difference that the nickel came from a single principal source, this being the copper ore from which the copper for the alloy was smelted. The association of the nickel with the copper in orichalcum is in- dicated by the tendency of the proportion of nickel to increase with an increase in the proportion of copper, as is shown in Table XXV for the coins analyzed in this present investigation. No correlation exists between the proportions of nickel and the proportions of any other component of orichalcum. The reason for the higher average proportion of nickel in the coins of Period II (Table XXIV) is therefore the higher average proportion of copper in the coins of this period. This association of nickel with copper has been noticed in other classes of ancient coins. 86 The nickel content of orichalcum coins is very similar to that of most other ancient coins composed of copper alloys that were issued in the Mediterranean region at various places and at various times. Thus for ancient Greek bronze coins in general 87 the average proportion of nickel has been found to be 0.04%, and for the local bronze coins of Athens struck in Roman Imperial times 88 the nickel content has been found to range from 0.02% to 0.06% with an average of 0.04%.
| Range of Copper Content % | Average Nickel Content % | Number of Coins |
| Under 76.00 | 0.015 | 2 |
| 76.01 to 80.00 | 0.018 | 9 |
| 80.01 to 84.00 | 0.035 | 6 |
| 84.01 to 88.00 | 0.037 | 6 |
| Over 88.00 | 0.040 | 2 |
Since cobalt is commonly associated with nickel in ores it might be expected that cobalt would also be present in orichalcum coins, though in lower proportions because of the lesser abundance of this element. Bibra (Table X) reported the presence of traces of cobalt in four of the coins he analyzed. No other previous analyst has reported its presence in orichalcum coins, probably because no tests were made for it. As will be seen from the notes to Table XVIII, traces of cobalt were found in five coins out of a total of twelve tested for this element. It is evident, therefore, that cobalt in very small proportions is frequently present as an accidental impurity in orichalcum.
Silver was found in all but five of the coins that were analyzed, but as will be seen from the results in Table XVIII the proportion did not exceed 0.10% in any one of the coins. The average proportion for the twenty coins that contained silver is only 0.05% and for the group as a whole only 0.04%. The proportions reported by Genth and by Helm (Tables IX and XI, respectively) are all higher. On the other hand, Bibra (Table X) reported silver to be present in only two coins, and then only in traces. The new results, which lie between these extremes, are probably nearer the truth.
The silver in orichalcum appears to be largely associated with the copper. This association is indicated by the tendency of the proportion of silver to increase with an increase in the proportion of copper, as is shown in Table XXVI for the coins analyzed in this investigation. No other regular correlation between the proportions of silver and the proportions of any other component is apparent from the data of Table XVIII, though it seems probable that some of the silver in some of the coins was associated with the lead. It seems significant that the four coins in which the proportion of lead is the lowest contain no silver, and that all of the four coins in which the proportion of lead is the highest contain silver, even though no regular correlation exists between the proportions of silver and lead in the coins that contain intermediate proportions of lead. Moreover, the coins of Period I in which the average proportion of lead is 0.23% contain only 0.02% silver on the average, whereas the coins of Period II in which the average proportion of lead is 1.06% contain 0.06% silver on the average. Thus it would appear that silver as an accidental impurity in orichalcum is sometimes associated with the lead as well as with the copper.
As shown in Table XVIII, only one coin of the group that was analyzed was found to contain a weighable proportion of gold, though traces were detected in nine others. The presence of gold in orichalcum has not been reported previously. This accidental impurity is probably associated with the copper, the silver, or both.
| Range of Copper Content % | Average Silver Content % | Number of Coins |
| Under 76.00 | 0.020 | 2 |
| 76.01–80.00 | 0.026 | 9 |
| 80.01–84.00 | 0.043 | 6 |
| 84.01–88.00 | 0.057 | 6 |
| Over 88.00 | 0.050 | 2 |
It is clear from Table XVIII that arsenic is a rather frequent minor component or impurity in orichalcum. This is contrary to the findings of previous investigators, none of whom reported the presence of arsenic except Bibra (Table X) and this investigator reported its presence in only three out of twenty-three coins, and then only as traces. Arsenic is a rather frequent impurity in Greek bronze coins, 89 the source apparently being copper ores, and its presence in orichalcum coins is therefore to be expected. From Table XVIII it will be seen that there is a decided tendency for arsenic to be present in higher proportions in late orichalcum coins. In the group of coins of Period I analyzed for this element, the average proportion is only 0.02%, whereas in the group of Period II, the average proportion is 0.09%.
As is evident from Table XVIII and the notes to this table, antimony occurs less frequently than arsenic, and in lower proportions. Antimony was found in only a third of the fifteen coins tested for this element, and the average proportion for this whole group of coins was only about 0.01%. This also is contrary to the findings of Bibra (Table X) who reported proportions of antimony ranging from 0.02% to 1.30% in eight of the coins he analyzed, and traces in nine others, out of a total of twenty-three. However, the method he employed for the detection and determination of antimony is known to be defective. 90 As with arsenic, there is a tendency for the proportion of antimony to be higher in later coins, though the evidence for this is perhaps less conclusive than for arsenic. Antimony was absent from six of the coins of Period I tested for this element, and the remaining two contained about 0.01% each, whereas it was absent from only four of Period II, and the remaining three were estimated to contain 0.01%, 0.05% and 0.10%, respectively.
Although only eight of the coins were tested for bismuth, the results of the tests indicated that this element is an infrequent trace impurity in orichalcum. The possible occurrence of bismuth in this alloy apparently has not been investigated before.
Sulfur is frequently present as an impurity in late orichalcum coins, but not in early ones, as may be seen from the data of Table XVIII. Of the ten coins of Period I tested for sulfur, nine were found to contain none, and the remaining one only 0.03%, whereas of the eight coins of Period II, only one was found to contain no sulfur, and in the remaining seven the proportion ranged from 0.08% to 0.32%. Furthermore, the later coins of Period II were found to contain the highest proportions. Bibra (Table X) found traces of sulfur in ten of the coins he analyzed, and 0.10% in each of two others. His results agree with the present results in that the measurable proportions of sulfur occurred in late coins. The likely sources of this impurity are sulfide ores of copper or zinc, or both.
As remarked previously, the defective summations of some of the analyses probably indicate the presence of oxygen in the form of metal oxides in certain of the coins. The microscopic examination of ancient alloys almost always reveals the presence of at least slight amounts of oxidized metal, but there is often uncertainty as to how much of this was present originally and how much was produced later by natural corrosion. However, in view of the crude methods of ancient metallurgy, the very frequent presence of at least traces of oxygen as an original accidental impurity in orichalcum appears very likely.
| 82 |
A detailed procedure for the determination of sulfur in ancient brass is given by Caley, E. R., Ohio Journal of Science, LXI (1961), pp. 151–154.
|
| 83 |
Harvey, C. E., A Method of Semi-Quantitative Spectrographic Analysis (Glendale, California, 1947).
|
| 84 |
Bibra, E. von, Die Bronzen und Kupferlegirungen der alten und ältesten Völker (Erlangen, 1869), pp. 1–2.
|
| 85 |
Desch, C. H., Report of the British Association for the Advancement of Science (1928), p. 437.
|
| 86 |
Caley, E. R., Chemical Composition of Parthian Coins, NNM, No. 129 (New York, 1955), p. 15.
|
| 87 |
Caley, E. R., The Composition of Ancient Greek Bronze Coins (Philadelphia, 1939), p. 154.
|
| 88 |
Caley, E. R., op.cit., p. 26.
|
| 89 |
Caley, E. R., The Composition of Ancient Greek Bronze Coins (Philadelphia, 1939), p. 161.
|
| 90 |
Ibid., p. 162.
|
Orichalcum may be defined as an alloy of copper and zinc in which the proportion of zinc is high enough to give it a color that is appreciably different from that of copper, and in which the proportion of any other metal, or combination of other metals, is considerably lower than that of the zinc. Of the other metals that may be present in ancient copper alloys containing zinc, only tin and lead are of significance as components that may be present in higher proportion than the zinc. In the early years of the Empire, as is shown by the analyses in the preceding tables, the proportion of zinc usually exceeds twenty per cent, and the proportion of tin and lead, taken together, usually amounts to but a few tenths of one per cent. Later, the proportion of zinc sometimes falls below ten per cent, and the proportion of tin or lead, or both together, often exceeds one per cent. The allowable lower limit for the proportion of zinc is to some extent a matter of arbitrary opinion. A reasonable conclusion would appear to be that an alloy containing less than five per cent of zinc should not be classed as orichalcum. The color of such an alloy of copper and zinc is not very different from that of copper itself, and zinc in such low proportion is more likely to be present as an accidental impurity in the copper than as an addition resulting from intentional manufacture. Furthermore, when the proportion of tin or lead, or both taken together, exceeds the proportion of zinc, the alloy also should not be classed as orichalcum since zinc is then no longer the principal alloying component. The Roman coinage alloys containing zinc that should not be classed under this term may be grouped as follows:
Class I Alloys containing less than five per cent of zinc, in which the proportion of tin or lead, or both together is less than the proportion of zinc;
Class II Alloys containing less than five per cent of zinc, in which the proportion of tin or lead, or both together, exceeds the proportion of zinc;
Class III Alloys containing five per cent or more of zinc, in which the proportion of tin or lead, or both together, exceeds the proportion of zinc.
When the proportions of zinc, tin and lead in an alloy of Class I amounts to only about one per cent, it may be regarded as being little more than an impure copper. An alloy of Class II may be termed a tin bronze when the proportion of tin is much higher than the proportions of zinc and lead, or a leaded tin bronze when the proportions of tin and lead are much higher than the proportion of zinc. An alloy of Class III may be termed a zinc bronze. Alloys of all three classes, with wide variations in the relative proportions of zinc, tin and lead, are well represented in the later Roman aes coinage.
On the basis of all the available analytical evidence, orichalcum is the only alloy that was used for the coinage of sestertii and dupondii in Period I, i.e., during the reigns from Tiberius to Nerva inclusive. This same evidence shows that it was almost exclusively used for this purpose in the first half of Period II, i.e., during the reigns from Trajan to Antoninus Pius inclusive. It is in the second half of this period that coins of these denominations were often struck in alloys other than orichalcum.
In Table XXVII are listed analyses by previous investigators of sestertii and dupondii of Period II composed of alloys which are not orichalcum in the proper meaning of this term. It will be seen that the metal of two of these coins (Nos. i and 4) belongs in Class I, the metal of five of them (Nos. 2, 5, 6, 7 and 8) in Class II, and the metal of the remaining one (No. 3) in Class III.
Orichalcum was rarely used for sestertii and dupondii in the third century. Analyses by previous investigators of coins of these denominations issued in the first half of this century are listed in Table
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Nickel % | Other % | Total % |
| 1 | 94.63 | 3.18 | 0.50 | 0.53 | 0.72 | 0.44 | trace | 100.00 |
| 2 | 83.39 | 0.85 | 6.20 | 6.79 | — | — | — | 97.23 |
| 3 | 79.24 | 6.29 | 4.99 | 9.20 | 0.23 | — | — | 99.95 |
| 4 | 92.57 | 4.01 | 1.80 | 0.25 | 1.24 | 0.10 | 0.03 | 100.00 |
| 5 | 82.81 | 4.86 | 3.97 | 8.36 | — | — | — | 100.00 |
| 6 | 88.88 | 3.82 | 4.20 | 2.60 | 0.50 | trace | trace | 100.00 |
| 7 | 89.41 | 0.94 | 5.42 | 4.23 | trace | trace | none | 100.00 |
| 8 | 87.23 | 4.50 | 3.55 | 4.40 | 0.21 | 0.11 | none | 100.00 |
Sestertius of Trajan. Wt. = 24.7 grams.
Said to contain traces of antimony and arsenic.
Analyzed by Bibra, Die Bronzen und Kupferlegirungen der alten und ältesten Völker (Erlangen, 1869), pp. 54–55, No. 38.
Sestertius of Marcus Aurelius.
Analysis published by Mattingly, BMC Coins of the Roman Empire, Vol. IV (London, 1940), p. xvi.
Sestertius of Faustina, Jr. Wt. = 23.5 grams.
Analyzed by Phillips, Journal of the Chemical Society, IV (1852), pp. 281–282.
Dupondius of Marcus Aurelius. Wt. = 9.2 grams.
Said to contain 0.03% antimony and traces of cobalt and arsenic. Analyzed by Bibra, op.cit., pp. 54–55, No. 58.
Dupondius of Marcus Aurelius.
Analysis published by Mattingly, op.cit., p. xvii.
The zinc content was merely estimated as being over 4%.
The figure in the table was obtained by difference.
Sestertius of Lucius Verus. Wt. = 23.7 grams.
Said to contain a trace of antimony.
Analyzed by Bibra, op.cit., pp. 54–55, No. 60.
Sestertius of Commodus. Wt. = 24.9 grams.
Analyzed by Bibra, op.cit., pp. 54–55, No. 64.
Dupondius of Crispina. Wt. = 11.4 grams.
Analyzed by Bibra, op.cit., pp. 54–55, No. 65.
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Nickel % | Other % | Total % |
| 1 | 86.98 | 5.00 | 4.27 | 3.23 | trace | 0.41 | 0.11 | 100.00 |
| 2 | 76.62 | 17.08 | 4.00 | 0.95 | 0.68 | 0.21 | 99.54 | |
| 3 | 71.56 | 8.79 | 6.45 | 13.09 | — | — | — | 99.89 |
| 4 | 70.83 | 6.90 | 5.97 | 16.29 | — | — | — | 99.99 |
| 5 | 75.00 | 5.63 | 6.82 | 12.00 | — | — | 0.05 | 99.50 |
| 6 | 84.00 | 3.35 | 6.33 | 6.00 | 0.30 | 0.02 | none | 100.00 |
| 7 | 84.49 | 3.15 | 5.98 | 6.15 | 0.13 | 0.10 | trace | 100.00 |
| 8 | 72.01 | 4.60 | 15.28 | 7.12 | — | — | — | 99.01 |
| 9 | 77.10 | 1.36 | 7.54 | 12.70 | — | — | — | 98.70 |
| 10 | 98.22 | none | 1.03 | 0.33 | trace | 0.30 | 0.12 | 100.00 |
| 11 | 97.52 | 0.67 | 0.53 | 0.48 | 0.40 | 0.40 | trace | 100.00 |
| 12 | 78.00 | 8.33 | 8.73 | 4.74 | trace | trace | 0.20 | 100.00 |
| 13 | 81.18 | 8.00 | 7.53 | 3.04 | 0.10 | 0.08 | 0.07 | 100.00 |
| 14 | 76.20 | 5.84 | 5.14 | 12.02 | — | — | — | 99.20 |
Dupondius of Caracalla. Wt. = 10.1 grams.
Said to contain 0.11% antimony and traces of cobalt and sulfur.
Analyzed by Bibra, op.cit., pp. 54–55, No. 66.
Sestertius of Julia Soaemias. Wt. = 23.6 grams.
Only the sum of the iron and nickel is reported.
Said to contain 0.21% silver.
Analyzed by Hofmann, Numismatische Zeitschrift, XVI (1884), p. 10.
Sestertius of Severus Alexander.
Analysis reported by Sabatier, Production de Vor, de Vargent et du cuivre chez les anciens et hôtels monétaires des romains et byzantins (Petersbourg, 1850), p. 77, No. 10.
Sestertius of Severus Alexander. Wt. = 16.4 grams.
Analyzed by Schardinger and reported by Hofmann, op.cit., p. 11.
Sestertius of Severus Alexander. Wt. = 18.8 grams.
Contains 0.05% of silver.
Analyzed by Hofmann, op.cit., p. 11.
Dupondius of Severus Alexander. Wt. = 11.0 grams.
Analyzed by Bibra, op.cit., pp. 54–55, No. 67.
Dupondius of Severus Alexander. Wt. = 11.5 grams.
Contains a trace of silver.
Analyzed by Helm, Zeitschrift für Ethnologie, XXVII (1895), p. 20.
Dupondius of Julia Mamaea.
Analysis reported by Sabatier, op.cit., p. 77, No. 12.
Sestertius of Gordian III.
Analysis reported by Sabatier, op.cit., p. 77, No. 13.
Sestertius of Gordian III. Wt. = 17.2 grams.
Said to contain 0.12% of antimony, and traces of arsenic and sulfur. Analyzed by Bibra, op.cit., pp. 56–57, No. 69.
Sestertius of Gordian III. Wt. = 16.2 grams.
Said to contain a trace of cobalt.
Analyzed by Bibra, op.cit., pp. 54–55, No. 68.
Sestertius of Gordian III. Wt. = 20.2 grams.
Said to contain 0.20% of antimony.
Analyzed by Bibra, op.cit., pp. 56–57, No. 70.
Dupondius of Gordian III. Wt. = 10.3 grams.
Said to contain 0.07% of antimony.
Analyzed by Bibra, op.cit., pp. 56–57, No. 71.
Sestertius of Philip I.
Analysis reported by Sabatier, op.cit., p. 77, No. 14.
Four new analyses of late sestertii are listed in Table XXIX. It will be seen that the metal of three of these coins belongs in Class II, and that of the remaining one in Class III.
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Nickel % | Other % | Total % |
| 1 | 80.45 | 3.99 | 4.96 | 9.62 | 0.51 | 0.07 | 0.14 | 99.74 |
| 2 | 84.97 | 4.42 | 7.56 | 2.67 | 0.28 | 0.04 | 0.09 | 100.03 |
| 3 | 79.10 | 0.83 | 6.42 | 13.37 | 0.03 | 0.16 | 0.06 | 99.97 |
| 4 | 67.67 | 7.58 | 6.61 | 16.96 | 1.08 | 0.03 | 0.11 | 100.04 |
Identifications and Notes
1. Emperor = Marcus Aurelius
Wt. = 23.6 grams
Date = a.d. 171–172
Size = 27–30 mm.
Condition = Fine
Obv.: Head of Marcus Aurelius, laureate, r.
M ANTONINVS AVG TRP XXVI
Rev.: Roma seated l., on cuirass, holding sceptre; her l. elbow resting on a round shield; behind her an oval shield.
IMP VI COS III In field, SC
Ref. BMC, Vol. IV, p. 622, No. 1420.
Analyst: W. H. Deebel
The minor components included 0.10% of silver, 0.04% of cobalt, and a trace of gold. Though the coin was fine externally, the metal was to some extent corroded internally, which accounts for the low summation.
2. Emperor = Marcus Aurelius
Wt. = 21.6 grams
Date = a.d. 177–178
Size = 28–30 mm.
Condition = Fair
Obv.: Head of Marcus Aurelius, laureate, r.
M AVREL ANTONINVS AVG TRP XXXII
Rev.: Felicitas standing l., holding caduceus and sceptre.
FELICITAS AVG IMP Vllll COS III PP
In field, S C
Ref. BMC, Vol. IV, p. 674, No. 1676, pi. 89, No. 2.
Analyst: W. H. Deebel
The minor components included 0.09% of silver and traces of cobalt and gold.
3. Emperor = Commodus
Wt. = 20.7 grams
Date = a.d. 189
Size = 27–30 mm.
Condition = Fair
Obv.: Head of Commodus, laureate, r.
M COMMOD ANT P FELIX AVG BRIT PP
Rev.: Minerva standing l., holding Victory and spear; shield l., trophy r.
MINER VICT PM TRP Xllll IMP VIII COS V DES VI
In field, S C
Ref. BMC, Vol. IV, p. 823, No. 637.
Analyst: W. H. Deebel
The minor components included 0.06% of silver and traces of cobalt and gold.
4. Emperor = Septimus Severus
Wt. = 21.4 grams
Date = a.d. 208
Size = 32 mm.
Condition = Poor
Obv.: Bust of Septimus Severus, laureate r.
Legend mostly illegible
Rev.: Emperor riding on horseback to 1.
Legend mostly illegible
Ref. BMC, Vol. V, p. 349.
Analysts: P. J. Elving and S. R. Ginsburg
The minor components included 0.10% of arsenic and 0.01% of sulfur.
Analyses of a few miscellaneous Roman coins containing zinc are listed in Table XXX. The first two are late asses. Early asses were almost always struck in nearly pure copper, but some exceptions are known, in particular during the reign of Nero when the as and even its fractions were struck in orichalcum for a brief period. 91 The metal of No. 1 belongs in Class I and that of No. 2 in Class II. No. 3 is of unusual composition for an Alexandrian bronze coin. Other analyses show that such coins were usually composed of a leaded tin bronze. 92 However, only a few coins of this class have been analyzed, and it is entirely possible that many such coins were issued in alloys that contained appreciable proportions of zinc. The metal of No. 4 may be classed as orichalcum, though the proportion of tin is unusually high for an alloy containing so much zinc. Its composition is not only unusual for a coin of so late a date, but even more unusual for a coin struck in a distant province. Analyses of late colonial and local coins of the Empire indicate that they were ordinarily struck in leaded tin bronze. 93 However, here again, not many analyses have been made, and it is entirely possible that some classes of these coins, at least at certain periods, were issued in alloys that contained zinc as a principal component.
| Coin No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Nickel % | Other % | Total % |
| 1 | 90.11 | 4.97 | 4.34 | 0.27 | 0.18 | 0.02 | 0.29 | 100.18 |
| 2 | 84.56 | 2.89 | 3.78 | 8.77 | — | — | — | 100.00 |
| 3 | 85.48 | 6.33 | 5.73 | 1.53 | 0.21 | 0.72 | trace | 100.00 |
| 4 | 79.27 | 16.33 | 2.71 | 0.73 | 0.43 | 0.53 | none | 100.00 |
Identifications and Notes
1. As of Faustina, Jr.
Wt. = 10.3 grams
Date = a.d. 161–176
Size = 25 mm.
Condition = Poor
Obv.: Bust of Faustina, Jr., to r.
Legend mostly illegible.
Rev.: Female figure standing with head to 1.
Legend mostly illegible.
Ref. BMC, Vol. IV, pp. 538–543.
Analysts: P. J. Elving and S. R. Ginsburg
The minor components included 0.06% arsenic and 0.23% sulfur.
2. As of Commodus. Analysis reported by Mattingly, loc.cit.
3. Alexandrian coin of Hadrian. Wt. = 8.0 grams.
Analyzed by Bibra, op.cit., pp. 84–85, No. 67.
4. Colonial coin of Elagabalus struck in Nicea in Bithynia. Analyzed by Bibra, op.cit., pp. 84–85, No. 68.
| 91 |
BMC Coins of the Roman Empire, Vol. I (London, 1923), pp. 248–259.
|
| 92 |
Caley, E. R., ANS Centennial Publication (New York, 1958), p. 176.
|
| 93 |
Caley, E. R., The Composition of Ancient Greek Bronze Coins (Philadelphia, 1939), pp. 26, 45, 92.
|
Sufficient data are now available from the results of previous analyses and new analyses to show the important variations in the composition of the orichalcum and zinc bronze sestertii and dupondii issued during the reigns of most of the principal emperors from the beginning of the Empire to about the middle of the third century. In order to show the significant variation in composition within the reign of any given emperor, and the variation from emperor to emperor, only the proportions of copper, zinc, tin and lead need be considered since the proportions of the other elements in the alloys do not vary in any systematic way. The proportions of these four principal metals in the coins of the individual emperors are shown in Tables XXXI to XLIX inclusive. In those tables which contain information on more than one coin, the analytical data are arranged in the order of the decreasing percentages of zinc in the coins. In the averages of all the analyses in each of these tables, the average proportions of tin and lead are given only if these elements were reliably determined in all the coins. The averages of what are termed "good analyses" in certain of the tables are based on the individual analyses which appear to be reliable from the standpoint of both the identification of the coins and the quality of the analyses.
The composition of the eight sestertii and dupondii of Augustus that have been analyzed is shown in Table XXXI. It would appear that such coins vary greatly in the proportions of copper and zinc they contain. However, the range of composition is much smaller if only the coins identified with certainty are considered. Nos. 1, 2, 3, 4 and 6 were coins of the IIIVIR type struck at an Italian mint or mints, and No. 5 was of the CA type struck at some eastern mint, but the types of Nos. 7 and 8 analyzed by Bibra (Table X, Nos. 1 and 2) are unknown since no descriptions accompanied his analyses. That they were of either the IIIVIR type or the CA type seems unlikely in view of their low zinc content. They may have been coins of certain of the many less common types struck under Augustus, or they may have been restored coins of Augustus struck at a much later date. The fact that their tin content is higher than that found in any other orichalcum coins struck prior to the middle of the first century a.d. would appear to be an indication of a later date. There is even a possibility that they were incorrectly identified and that they were not coins of Augustus at all. However this may be, it is clear from what is now known about the composition of the sestertii and dupondii struck prior to the middle of the first century a.d. that the composition of coins of the IIIVIR type set a pattern or standard for the composition of the orichalcum used for the coins of these two denominations for over half a century after the first issue of coins of this type. Although the data on the tin and lead content of the orichalcum coins of Augustus are insufficient to allow any exact conclusion as to the ranges and average proportions of these metals, it seems certain that both were present merely as accidental impurities. Since only five IIIVIR type coins and only a single CA type coin have been analyzed up to now, more analyses of coins of these important types, and analyses of coins of certain other types as well, are needed before any definitive conclusions can be reached about the composition of the orichalcum coins of Augustus.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Dupondius | 76.4 | 23.6 | — | — |
| 2 | Sestertius | 76.7 | 23.3 | — | — |
| 3 | Sestertius* | 76.70 | 22.02 | 0.27 | 0.37 |
| 4 | Dupondius* | 77.36 | 21.88 | 0.17 | 0.16 |
| 5 | Dupondius | 78.7 | 20.6 | — | 0.7 |
| 6 | Sestertius | 82.38 | 17.36 | — | — |
| 7 | Sestertius | 87.05 | 11.80 | 0.72 | trace |
| 8 | Sestertius | 92.57 | 5.15 | 1.05 | trace |
| Av. of all analyses | 81.0 | 18.2 | — | — | |
| Av. of new analyses* | 77.03 | 21.95 | 0.22 | 0.27 | |
| Av. of IIIVIR type coins | 77.9 | 21.6 | — | — |
Unfortunately, only a single analysis, that listed in Table XXXII, of an orichalcum coin of Tiberius is available. However, since the composition of this coin agrees well with the composition of the orichalcum coins of both his predecessor and his successor, it is not unlikely that its composition is typical of the composition of the sestertii and dupondii of this emperor.
| Copper % | Zinc % | Tin % | Lead % |
| 76.86 | 22.85 | 0.03 | 0.04 |
The composition of the six sestertii and dupondii of Caligula that have been analyzed is shown in Table XXXIII. The zinc content of No. 1 is the highest that has been found in any Imperial orichalcum coin that has been carefully analyzed. However, the average proportions of copper and zinc in the coins of Caligula are very close to the average proportions of these metals in the IIIVIR type coins of Augustus. The proportions of tin and lead in these coins of Caligula are remarkably low, and with the exception of the single coin of Tiberius listed in Table XXXII, are in fact lower than the proportions of these metals in the orichalcum coins of any other emperor.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Dupondius* | 72.63 | 26.71 | 0.02 | 0.16 |
| 2 | Sestertius* | 77.52 | 22.20 | 0.04 | 0.01 |
| 3 | Sestertius* | 78.19 | 21.11 | 0.12 | 0.04 |
| 4 | Dupondius | 79.3 | 20.7 | — | — |
| 5 | Dupondius | 80.3 | 19.7 | — | — |
| 6 | Sestertius* | 81.03 | 18.55 | none | 0.05 |
| Av. of all analyses | 78.2 | 21.5 | — | — | |
| Av. of new analyses* | 77.34 | 22.14 | 0.05 | 0.07 |
In Table XXXIV is shown the composition of the eight sestertii and dupondii of Claudius that have been analyzed. The zinc content of No. 1 is the highest that has been reported for any orichalcum coin of the Imperial period, but this result must be regarded with some doubt because the method used by the analyst could not have yielded a very accurate result. The closeness of the average proportions of copper and zinc to those in the coins of Caligula is remarkable. The proportions of tin and lead are definitely higher and more erratic.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Dupondius | 72.20 | 27.7 | — | — |
| 2 | Sestertius* | 75.91 | 23.20 | 0.09 | 0.12 |
| 3 | Sestertius | 77.9 | 22.1 | — | — |
| 4 | Sestertius | 77.44 | 21.50 | 0.30 | trace |
| 5 | Sestertius | 76.85 | 21.33 | — | 0.20 |
| 6 | Dupondius* | 77.59 | 21.11 | 0.10 | 0.83 |
| 7 | Dupondius | 81.4 | 18.6 | — | — |
| 8 | Dupondius | 81.1 | 15.7 | — | — |
| Av. of all analyses | 77.5 | 21.4 | — | — | |
| Av. of new analyses* | 76.75 | 22.15 | 0.10 | 0.48 |
The composition of a sestertius and three dupondii of Nero is shown in Table XXXV. It will be seen that the average proportion of zinc is lower than in the coins of the preceding emperors. Though a final
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Dupondius* | 77.27 | 22.46 | none | 0.15 |
| 2 | Dupondius* | 78.24 | 20.98 | none | 0.14 |
| 3 | Sestertius | 81.07 | 17.82 | 1.05 | — |
| 4 | Dupondius* | 83.16 | 15.95 | 0.01 | 0.12 |
| Av. of all analyses | 79.94 | 19.30 | — | — | |
| Av. of new analyses* | 79.56 | 19.80 | trace | 0.14 |
In Table XXXVI is shown the composition of a sestertius and a dupondius of Vespasian. No averages are given because there are only two analyses, and one of them is not very exact. However, it is evident that the zinc content of the coins is generally lower than that of the coins of Nero, and the proportions of tin and lead generally higher. If the decline in the quality of orichalcum did not begin with the reign of Nero it certainly did with the reign of Vespasian.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Sestertius | 81.4 | 16.4 | 0.8 | 1.1 |
| 2 | Dupondius | 85.89 | 13.02 | 0.40 | 0.31 |
The composition of dupondii of Titus and Domitian is shown in Table XXXVII. Their zinc content is definitely lower than that of the two coins of Vespasian, though this difference is perhaps not significant in view of the small number of analyses on which to base this conclusion. With the exception of one coin of Augustus of somewhat uncertain identity, the zinc content of No. 2 is the lowest that has been found in any orichalcum coin issued prior to the second century a.d.
| No. | Emperor | Copper % | Zinc % | Tin % | Lead % |
| 1 | Titus | 83.13 | 15.90 | — | — |
| 2 | Domitian | 88.19 | 10.23 | 0.51 | 0.30 |
The composition of three dupondii of Nerva is shown in Table XXXVIII. They are very similar in composition, which is not surprising in view of the fact that they must all have been issued within a period of about two years. Their average zinc content is only slightly less than the average zinc content of the four coins of Vespasian, Titus and Domitian listed in the two preceding tables. The proportions of tin and lead in the orichalcum coins issued during the reigns of Vespasian to Nerva inclusive are generally higher than in the coins of preceding emperors.
| No. | Copper % | Zinc % | Tin % | Lead % |
| 1 | 83.60 | 14.82 | 0.70 | 0.54 |
| 2* | 84.69 | 13.59 | 0.57 | 0.49 |
| 3* | 86.30 | 12.94 | 0.52 | trace |
| Av. of all analyses | 84.86 | 13.78 | 0.60 | 0.34 |
| Av. of new analyses* | 85.50 | 13.27 | 0.55 | 0.25 |
As is shown in Table XXXIX, a considerable amount of information is available on the composition of the sestertii and dupondii of Trajan. In these coins as a whole the proportions of copper and zinc vary over a wide range. However, if Nos. 13, 14, 15 and 16 are excluded, the range of variation of the proportions of copper and zinc in the remainder, constituting seventy-five per cent of the coins, is not very great. The composition of the coins of this large fraction may perhaps be regarded as typical for the reign of this emperor. The zinc content of No. 16 is so low that the alloy is better classified as impure copper rather than orichalcum. This coin is clearly atypical in composition. In most of the individual coins, and on the average, the proportions of tin and lead are much higher than in the coins of any preceding emperor. The proportions of both tin and lead in No. 10 are unusually high for a coin of this period, and for this reason this coin is also atypical in composition.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Dupondius* | 81.21 | 17.76 | 0.02 | 0.37 |
| 2 | Dupondius | 78.08 | 16.68 | 2.14 | 0.57 |
| 3 | Sestertius | 79.5 | 16.6 | trace | 1.3 |
| 4 | Dupondius | 82.2 | 16.5 | 0.5 | 0.8 |
| 5 | Dupondius | 83.4 | 16.4 | — | trace |
| 6 | Sestertius | 80.6 | 16.4 | 3.0 | — |
| 7 | Dupondius* | 83.48 | 16.01 | 0.02 | 0.10 |
| 8 | Sestertius | 80.09 | 15.45 | 2.28 | 1.63 |
| 9 | Sestertius | 82.13 | 15.35 | 1.12 | trace |
| 10 | Dupondius* | 76.83 | 14.45 | 4.34 | 4.33 |
| 11 | Sestertius* | 82.08 | 14.10 | 2.05 | 1.29 |
| 12 | Sestertius | 85.3 | 13.9 | 0.8 | — |
| 13 | Dupondius | 83.95 | 12.42 | 2.22 | 0.30 |
| 14 | Sestertius | 87.12 | 9.90 | 2.13 | 0.48 |
| 15. | ? | 88.58 | 7.56 | 1.80 | 2.28 |
| 16 | Sestertius | 94.63 | 3.18 | 0.50 | 0.53 |
| Av. of all analyses | 83.0 | 13.9 | — | — | |
| Av. of new analyses* | 80.90 | 15.58 | 1.61 | 1.52 | |
| Av. of good analyses | 83.46 | 12.99 | 1.69 | 1.08 |
A considerable amount of information is also available on the composition of the sestertii and dupondii of Hadrian, as is shown in Table XL. In these coins also, the proportions of copper and zinc vary over a wide range, but on the average the zinc content is decidedly lower than in the coins of Trajan. The average proportions of tin and lead are also lower. There appears to be no basis for classifying any of these coins as being abnormal or atypical in composition.
The composition of ten sestertii and dupondii of Antoninus Pius is shown in Table XLI. It will be seen that the ranges in the proportions of copper and zinc are smaller than in the coins of Hadrian, and that the proportions of zinc are lower at both ends of the scale. However, the average proportion of zinc is only a little less than in the coins of Hadrian. The proportions of tin and lead are also similar.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Sestertius | 82.35 | 16.84 | 0.43 | trace |
| 2 | Dupondius | 82.91 | 15.57 | 0.60 | 0.06 |
| 3 | Sestertius | 84.8 | 14.8 | trace | trace |
| 4 | Sestertius | 85.14 | 13.98 | 0.68 | 0.12 |
| 5 | Dupondius | 85.7 | 13.6 | — | — |
| 6 | Sestertius | 86.5 | 13.5 | — | — |
| 7 | Sestertius | 86.1 | 13.4 | 0.2 | — |
| 8 | Dupondius | 83.7 | 12.7 | 2.8 | 0.8 |
| 9 | ? | 86.92 | 10.97 | 0.72 | 1.10 |
| 10 | Sestertius | 85.77 | 10.89 | 1.15 | 1.74 |
| 11 | Dupondius | 88.50 | 9.05 | 1.27 | 0.30 |
| 12 | Sestertius | 91.24 | 7.14 | 0.32 | 0.44 |
| 13 | Sestertius | 90.49 | 7.04 | 1.10 | 0.20 |
| 14 | Sestertius | 89.92 | 6.74 | 1.52 | 0.37 |
| Av. of all analyses | 87.4 | 11.9 | — | — | |
| Av. of good analyses | 87.03 | 10.91 | 0.87 | 0.48 |
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Sestertius | 86.39 | 13.1 | — | trace |
| 2 | Dupondius* | 86.28 | 12.71 | 0.06 | 0.32 |
| 3 | Sestertius | 86.85 | 12.6 | — | trace |
| 4 | Sestertius* | 86.79 | 12.33 | none | 0.13 |
| 5 | Sestertius | 87.88 | 11.28 | none | 0.09 |
| 6 | Dupondius* | 86.51 | 11.14 | 1.69 | 0.11 |
| 7 | Dupondius* | 89.29 | 9.38 | 0.16 | 0.35 |
| 8 | Sestertius | 87.86 | 8.14 | 3.88 | trace |
| 9 | Dupondius | 92.79 | 6.7 | trace | trace |
| 10 | Sestertius | 91.72 | 5.33 | 1.55 | trace |
| Av. of all analyses | 88.2 | 10.3 | — | — | |
| Av. of new analyses* | 87.22 | 11.39 | 0.48 | 0.23 | |
| Av. of good analyses | 88.05 | 10.04 | 1.05 | 0.14 |
The composition of sixteen sestertii and dupondii of Marcus Aurelius and Lucius Verus is shown in Table XLII. It is evident that these coins as a group are radically different in composition from those of Antoninus Pius and from those of all the preceding emperors as well. Their average zinc content is much lower, and the average proportions of tin and lead much higher. Only half the coins are composed of orichalcum, the remainder being composed of zinc bronze, except for No. 16 in which the alloy is a leaded tin bronze.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Sestertius | 88.71 | 10.8 | trace | trace |
| 2 | Dupondius | 88.59 | 10.4 | trace | 0.48 |
| 3 | Sestertius | 81.47 | 10.30 | 6.62 | 0.02 |
| 4 | Sestertius* | 87.86 | 9.06 | 2.03 | 0.23 |
| 5 | Dupondius* | 88.96 | 7.87 | 2.33 | 0.18 |
| 6 | Sestertius | 87.73 | 7.5 | 1.58 | 2.71 |
| 7 | Sestertius | 87.31 | 7.08 | 4.02 | 0.83 |
| 8 | Sestertius | 89.13 | 7.0 | 3.33 | trace |
| 9 | Sestertius | 79.24 | 6.29 | 4.99 | 9.20 |
| 10 | Sestertius | 85.63 | 6.07 | 4.62 | 2.00 |
| 11 | Dupondius | 87.47 | 5.4 | 5.06 | 1.56 |
| 12 | Dupondius | 82.81 | 4.5 | 3.97 | 8.36 |
| 13 | Sestertius* | 84.97 | 4.42 | 7.56 | 2.67 |
| 14 | Sestertius* | 80.45 | 3.99 | 4.96 | 9.62 |
| 15 | Sestertius | 88.88 | 3.82 | 4.20 | 2.60 |
| 16 | Sestertius | 83.39 | 0.85 | 6.20 | 6.79 |
| Av. of all analyses | 85.79 | 6.6 | 3.84 | 2.95 | |
| Av. of new analyses* | 85.56 | 6.34 | 4.22 | 2.01 | |
| Av. of good analyses | 84.82 | 5.98 | 4.75 | 3.41 |
As is shown in Table XLIII, the average zinc content of eight sestertii and dupondii of Commodus is even less than that of the coins of Marcus Aurelius and Lucius Verus. Their tin content is about the same, but their average lead content is higher, though this is partly due to the very high lead content of No. 8. In this group also, only half the coins are composed of orichalcum. The other half is equally divided between coins composed of zinc bronze and coins composed of leaded tin bronze.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Dupondius | 78.24 | 10.2 | 3.12 | 7.98 |
| 2 | Sestertius | 87.70 | 7.92 | 2.90 | 0.42 |
| 3 | Sestertius | 87.07 | 7.1 | 1.94 | 3.37 |
| 4 | Dupondius | 86.85 | 6.43 | 1.64 | 4.28 |
| 5 | Sestertius | 82.69 | 5.8 | 2.93 | 8.12 |
| 6 | Sestertius | 85.60 | 5.77 | 4.02 | 4.17 |
| 7 | Sestertius | 89.41 | 0.94 | 5.42 | 4.23 |
| 8 | Sestertius | 79.10 | 0.83 | 6.42 | 13.37 |
| Av. of all analyses | 84.58 | 5.6 | 3.55 | 5.74 | |
| Av. of good analyses | 85.73 | 4.38 | 4.08 | 5.29 |
Unfortunately, very few analyses are available of sestertii and dupondii issued by the emperors between Commodus and Severus Alexander. The composition of a sestertius of Septimus Severus is shown in Table XLIV. The alloy of this coin may be classed as a zinc bronze containing a very high proportion of lead. It is, in fact, the highest proportion of lead ever found in any sestertius or dupondius. Shown in Table XLV is the composition of a dupondius of Caracalla. The alloy of this coin is also a zinc bronze, but one containing a low proportion of lead. The composition of a sestertius of Julia Soaemias is shown in Table XLVI. This is of special interest because it is apparently the latest Roman coin of regular Imperial issue composed of an alloy that may be classed as orichalcum. The colonial coin of Elagabalus of similar composition listed as No. 4 of Table XXX must be contemporaneous or nearly so. The fact that these two coins of the same regime are composed of orichalcum containing a moderately high proportion of zinc suggests that this type of orichalcum may have been commonly used for a brief period at this late date. However, no valid generalization about the composition of the sestertii or dupondii issued between the reigns of Commodus and Severus Alexander can be based on these isolated examples, except possibly to conclude that they vary greatly in composition.
| Copper % | Zinc % | Tin % | Lead % |
| 67.67 | 7.58 | 6.61 | 16.96 |
| Copper % | Zinc % | Tin % | Lead % |
| 86.98 | 5.00 | 4.27 | 3.23 |
| Copper % | Zinc % | Tin % | Lead % |
| 76.62 | 17.08 | 4.00 | 0.95 |
Shown in Table XLVII is the composition of six sestertii and dupondii of Severus Alexander. The alloys of all these coins may be classed as zinc bronzes, but the first three contain high proportions of lead and the other three much lower proportions. No. 4 is remarkable as containing the highest proportion of tin ever found in a sestertius or dupondius.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Sestertius | 71.56 | 8.79 | 6.45 | 13.09 |
| 2 | Sestertius | 70.83 | 6.90 | 5.97 | 16.29 |
| 3 | Sestertius | 75.00 | 5.63 | 6.82 | 12.00 |
| 4 | Dupondius | 72.01 | 4.60 | 15.28 | 7.12 |
| 5 | Dupondius ? | 84.00 | 3.35 | 6.33 | 6.00 |
| 6 | Dupondius ? | 84.49 | 3.15 | 5.98 | 6.15 |
| Av. of all analyses | 76.32 | 5.40 | 7.81 | 10.11 |
The composition of four sestertii and a dupondius of Gordianus III is shown in Table XLVIII. It will be seen that these coins vary more in composition than those of Severus Alexander. Nos. 1 and 2 are composed of zinc bronze, and No. 3 of leaded tin bronze, but the metal of the last two is only impure copper.
| No. | Denomination | Copper % | Zinc % | Tin % | Lead % |
| 1 | Sestertius | 78.00 | 8.33 | 8.73 | 4.74 |
| 2 | Dupondius | 81.18 | 8.00 | 7.53 | 3.04 |
| 3 | Sestertius | 77.10 | 1.36 | 7.54 | 12.70 |
| 4 | Sestertius | 97.52 | 0.67 | 0.53 | 0.48 |
| 5 | Sestertius | 98.22 | none | 1.03 | 0.33 |
| Av. of all analyses | 86.40 | 3.67 | 5.07 | 4.26 |
A sestertius of Philip I is the latest Roman coin of any denomination in which zinc has been found to be an essential component of the alloy. As is shown in Table XLIX, the alloy is a zinc bronze containing a high proportion of lead, and is very similar in composition to the alloy of a sestertius of Severus Alexander listed as No. 3 of Table XLVII.
| Copper % | Zinc % | Tin % | Lead % |
| 76.20 | 5.84 | 5.14 | 12.02 |
In all the preceding discussion, the sestertii and dupondii have been considered together on the assumption that the coins of each of these denominations issued under the same emperor were very similar in composition. The extent to which this is true is indicated by the data in Table L, in which are listed the average proportions of copper and zinc in the sestertii and dupondii of those emperors for which a sufficient number of examples of the coins of each denomination have been analyzed to make such a comparison possible. Though the data appear to show that the average copper content of the sestertii is more often a little higher and the average zinc content a little lower than in the dupondii, the difference is rather slight on the whole, for the average of all the averages for the copper content of the sestertii is 82.6%, and that for the dupondii is 82.1%. Also, the average of all the averages for the zinc content of the sestertii is 15.0%, and that for the dupondii is 15.9%. Even such small differences might become still smaller if larger numbers of sestertii and dupondii were analyzed and compared in composition. There appears to be no reasonable doubt that the intention was to strike Imperial sestertii and dupondii in orichalcum of the same composition in each of the individual reigns over the long period when this alloy was used exclusively as the material for the coins of these denominations.
| Emperor | Denomination | Copper % | Zinc % |
| Augustus | Sestertius* | 78.6 | 20.9 |
| Dupondius* | 76.9 | 22.8 | |
| Caligula | Sestertius | 78.9 | 20.6 |
| Dupondius | 77.4 | 22.5 | |
| Claudius | Sestertius | 77.0 | 22.0 |
| Dupondius | 78.1 | 20.8 | |
| Trajan | Sestertius | 83.9 | 13.1 |
| Dupondius | 81.3 | 15.8 | |
| Hadrian | Sestertius | 86.9 | 11.6 |
| Dupondius | 85.2 | 12.7 | |
| Antoninus Pius | Sestertius | 87.9 | 10.5 |
| Dupondius | 88.7 | 10.0 | |
| Marcus Aurelius | Sestertius | 85.4 | 6.4 |
| and Lucius Verus | Dupondius | 87.0 | 7.0 |
| *IIIVIR type |
Two salient facts emerge from this survey of the composition of sestertii and dupondii. One is the almost constant average zinc con- tent of the coins for something over half a century after the first IIIVIR type coins of Augustus were issued. The other is the systematic decrease in the zinc content after this initial period. The data show that the average proportion of zinc begins to decrease with the reign of Nero, and then continues to decrease at various rates. This change is rapid until the time of Nerva, is more gradual during the reigns of Trajan, Hadrian and Antoninus Pius, and then again undergoes a sharp decrease in the joint reign of Marcus Aurelius and Lucius Verus, followed by a smaller decrease in the reign of Commodus. For those reigns from Augustus to Commodus, inclusive, for which sufficient data are available, the most reliable figures for the average zinc content are listed in Table LI. The figures for Period I are based on the new analyses, and those for Period II on the "good" analyses. As is also shown in Table LI, the systematic changes in zinc content are accompanied by corresponding changes in the average proportions of tin and lead in the coins. These averages are on the same basis as the averages for zinc. Though the changes in the average proportion of tin or lead, or both taken together, are much less regular than the changes in the average proportions of zinc, certain general trends are obvious. In Period I the proportions of tin, lead, or both, are generally lower than in Period II. Within Period I, there are two reigns, those of Caligula and Nero, in which the proportions of these metals in the alloy are very low. At the end of this period, in the reign of Nerva, the coins have a higher average tin content, and a higher total content of tin and lead than in any previous reign. In the reign of Trajan the average proportions of these metals increase markedly. Though the tin content is lower in the two succeeding reigns, it is higher than in any reign of Period I, as is also the total content of tin and lead. With the joint reign of Marcus Aurelius and Lucius Verus there is a sudden relatively large increase in the proportions of both tin and lead, followed by a marked increase in the proportion of lead in the reign of Commodus. At the end of Period II, both the average lead content and the total tin and lead content exceed the average proportion of zinc in the coins. Possible reasons for these more or less systematic chronological changes in the zinc, tin and lead content of the sestertii and dupondii are discussed in the next section.
| Period | Emperor | Zinc % | Tin % | Lead % | Tin+Lead % |
| I | Augustus | 21.95 | 0.22 | 0.27 | 0.49 |
| Caligula | 22.14 | 0.05 | 0.07 | 0.12 | |
| Claudius | 22.15 | 0.10 | 0.48 | 0.58 | |
| Nero | 19.80 | trace | 0.14 | 0.14 | |
| Nerva | 13.27 | 0.55 | 0.25 | 0.80 | |
| II | Trajan | 12.99 | 1.69 | 1.08 | 2.77 |
| Hadrian | 10.91 | 0.87 | 0.48 | 1.35 | |
| Antoninus Pius | 10.04 | 1.05 | 0.14 | 1.19 | |
| Marcus Aurelius and Lucius Verus | 5.98 | 4.75 | 3.41 | 8.16 | |
| Commodus | 4.38 | 4.08 | 5.29 | 9.37 |
All the available evidence indicates that the manufacture of orichalcum in Roman times began about 45 b.c., and that its sole use in the very late Republican period was for coinage. No orichalcum objects other than coins datable to this period have been found, and literary evidence for any other use is lacking. Grant 94 is of the opinion that its manufacture during this period was a monopoly of the state for exclusive use in coinage. A few miscellaneous orichalcum objects appear to date from as early as the first half century of the Imperial period and a small number of objects other than coins have been found that are of later date, but there are various indications that coins served as the source of the metal for most, if not all, of these miscellaneous objects. The possibility exists, therefore, that the Roman state continued to exercise a monopoly on the manufacture of orichalcum, for it is not easy to account otherwise for its exclusive, or almost exclusive, use for coinage.
No details of the process used for the manufacture of orichalcum by the Romans have come down to us. As with most other ancient technical processes, those who knew the details left no records and those who wrote about such processes had no first-hand information. Moreover, the writers of the time may not have been able to obtain much information about this particular process because it was a monopoly of the state and an integral part of government minting practice. However, it is possible for us to gain some insight into this manufacturing process from some remarks made by Pliny and by Dioscorides, from the results of analyses of orichalcum coins, and from certain chemical and metallurgical considerations.
Pliny, 95 in the course of his discussion of the relative merits of different kinds of copper, makes the following pertinent remark:
Velocis defectus Livianum quoque, certe admodum exiguum invenitur, summa gloriae nunc in Marianum conversa, quod et Cordubense dicitur, hoc a Liviano cadmeam maxime sorbet et aurichalei bonitatem imitatur in sestertiis dupondiariisque, Cyprio suo assibus contentis.
(Livian copper also gave out quickly, for very little is now found. The highest reputation has now gone to the Marian kind, also called the Cordovan. Next to the Livian, this kind most readily absorbs cadmea and reproduces the excellence of aurichalcum in making sestertii and dupondii. The as must still be content with its own Cyprian copper). The word cadmea has been only transliterated since there is no exact English equivalent. From the descriptions of Pliny 96 and of Dios-corides, 97 the Latin cadmea and the corresponding Greek кαδμεíα were, in the first century a.d., names that denoted two distinct groups of related substances. The first group included certain zinc minerals, probably only calamine and smithsonite, and the ores composed chiefly of one mineral or the other. Some statements of Pliny would appear to indicate that one kind of copper ore was also included, but this would seem to be one of his not uncommon errors. The second group included artificial products obtained as sublimates in the flues and on the walls of smelting furnaces, but these were sometimes prepared by roasting one of the substances in the first group separately in a special furnace. These artificial products appear always to have been kinds of zinc oxide that differed only in physical form and in degree of purity. Some of them were given special names based on their superficial appearance. Natural cadmea appears to have been the kind used to treat copper in order to convert it into orichalcum, but there is a possibility that the artificial products were sometimes used.
The statement of Pliny quoted above leaves no doubt that orichalcum was produced for Roman coins by a cementation process. In this type of process for the manufacture of brass as carried out in medieval and early modern time, 98 bars, plates, or irregular pieces of copper were buried in a mixture of zinc ore and charcoal in a crucible, and on heating the charge to a sufficiently high temperature the zinc ore was reduced by the charcoal and the liberated metal in the form of a vapor was then absorbed by the copper. The degree of penetration of the copper by the zinc and the total proportion of zinc absorbed was dependent on the surface area of the copper, the thickness of the copper, the temperature, and the length of time of treatment, but it was not possible by this treatment alone to produce a uniform alloy. In order to produce such an alloy the temperature was raised to the fusion point and the molten metal was throughly stirred. A remark by Dioscorides 99 indicates that the Roman process was different. He states that in the final treatment of copper the founders threw on it large portions of finely ground кαδμεíα in order to improve the quality of the metal. Since treatment with zinc ore or zinc oxide alone would have had no effect, some reducing agent such as charcoal must also have been present. Possibly the actual procedure was to stir both charcoal and кαδμεíα into the molten copper in the form of a mixture of the two. Another possibility is that only a zinc mineral or a zinc ore was added, and that this was stirred into the molten copper with wooden poles which supplied the necessary reducing agents. Although a copper-zinc alloy was undoubtedly produced by one procedure or the other, a considerable proportion of the zinc ore or zinc oxide must have been lost by volatilization. An indication of this is the mention by Dioscorides of the very fine white smoke (i.e., zinc oxide) that was evolved during the treatment of the copper. The quantity of this was in fact so great that it was worth while to collect it from the flues and walls of the melting furnace. Not only was this process for the manufacture of orichalcum wasteful of the zinc mineral or ore, but the proportion of zinc in the finished alloy must have been difficult to control. However, neither the metalworkers nor anyone else at that time had the idea that zinc was alloyed with copper in the process, and that the color and other properties varied with the proportion of zinc. The statements of both Pliny and Dioscorides show that they had no understanding of the chemical changes involved in the treatment of copper with compounds containing zinc. Pliny apparently believed that the metal absorbed the cadmea, as is evident from the preceding quotation, and Dioscorides believed that it merely improved the quality of the copper.
That the Roman process did not always produce a homogeneous alloy is obvious from physical examination of the metal of sestertii and dupondii. Sometimes the degree of heterogeneity is so great that it is evident from visual inspection of the coins themselves, but in a larger proportion, only when sections of metal cut from coins are examined with a microscope by the technique of metallography. One occurrence of a very marked degree of heterogeneity was encountered in the course of the present investigation. A sestertius of Caligula, while being sampled for analysis, was observed to contain an enclosed irregular lump of metal that was reddish in color in sharp contrast with the bright yellow color of the bulk of the metal. This lump was isolated and analyzed. Its composition as compared with that of the bulk of the metal is shown in Table LII. The low summation of the analysis is due to the presence of cuprous oxide, which, in addition to the low zinc content, fully accounts for the observed reddish color.
| Metal | Inclusion % | Remainder % |
| Copper | 92.14 | 77.52 |
| Zinc | 6.65 | 22.20 |
| Tin | 0.06 | 0.04 |
| Lead | 0.02 | 0.01 |
| Iron | 0.24 | 0.27 |
| Nickel | 0.03 | 0.03 |
| —— | —— | |
| Total | 99.14 | 100.07 |
Analyst: M. C. Suarez
A rather close estimate of the purity of the copper used in the manufacture of orichalcum may be obtained from the data of the new analyses of this investigation. As previously explained, the impurities found on analysis were largely, if not entirely, associated with the copper. If the assumption is made that all these impurities were associated with the copper and none with the zinc, it is possible to calculate the purity of the copper used in the manufacturing process simply by prorating the percentages of copper and total impurities over a scale of one hundred percent. The results of such calculations for the coins of Period I are shown in Table LIII. Since the contemporaneous as was struck from ordinary unalloyed copper, in all probability of the same quality as that used for making orichalcum, a comparison of the results in this table with figures for the purity of the copper of the as should be a means of checking the validity of the above assumption. Unfortunately, only a few careful quantitative analyses have been made of examples of the Roman as. Some of the early analysts of coins of this denomination reported that they were composed of completely pure copper, and this erroneous information is frequently found in works on Roman numismatics. The only determinations of the actual purity of the metal sufficiently accurate for the present comparison are listed in Table LIV, and even some of these were obtained by making certain corrections in the published analytical data, as is stated in the notes to this table. In correcting the
| Coin No. | Emperor | Copper % | Total Impurities % |
| 1 | Augustus | 99.05 | 0.95 |
| 2 | Augustus | 98.45 | 1.55 |
| Av. = 98.75 | 1.25 | ||
| 3 | Tiberius | 99.53 | 0.47 |
| 4 | Caligula | 99.05 | 0.95 |
| 5 | Caligula | 99.52 | 0.48 |
| 6 | Caligula | 99.30 | 0.70 |
| 7 | Caligula | 99.63 | 0.37 |
| Av. = 99.38 | 0.62 | ||
| 8 | Claudius | 98.76 | 1.24 |
| 9 | Claudius | 98.14 | 1.86 |
| Av. = 98.45 | 1.55 | ||
| 10 | Nero | 99.25 | 0.75 |
| 11 | Nero | 99.43 | 0.57 |
| 12 | Nero | 99.45 | 0.55 |
| Av. = 99.38 | 0.62 | ||
| 13 | Nerva | 97.94 | 2.06 |
| 14 | Nerva | 98.25 | 1.75 |
| Av. = 98.10 | 1.90 |
| Coin No. | Emperor | Copper % | Total Impurities % |
| 1 | Augustus | 98.73 | 1.27 |
| 2 | Tiberius | 99.65 | 0.35 |
| 3 | Caligula | 99.24 | 0.76 |
| 4 | Caligula | 99.11 | 0.89 |
| 5 | Nero | 99.10 | 0.90 |
| 6 | Vespasian | 99.74 | 0.26 |
| 7 | Domitian | 99.33 | 0.67 |
Notes
Nos. 1 and 2 were analyzed by P. J. Elving and S. R. Ginsburg and were published in Caley, E. R., The Composition of Ancient Greek Bronze Coins (Philadelphia, 1939), p. 107. The analysis of No. 1 has been corrected for the low summation due to the presence of oxygen. The remainder were analyzed by E. von Bibra and were published in his Die Bronzen und Kupferlegirungen der alten und ältesten Völker (Erlangen, 1869), pp. 52–53. The analyses of Nos. 4 to 7 inclusive have been corrected for the high nickel results obtained by the method used by Bibra, and the analyses of Nos. 5 and 6 for the supposed antimony content reported by Bibra.
The rather wide variations in the percentages of zinc in the coins of even the earlier emperors (Tables XXXI, XXXIII and XXXIV) are easily understandable in view of the difficulty of controlling the zinc content closely in the process used for the manufacture of the alloy. Nevertheless, the average ratio of the proportion of zinc to the proportion of copper is remarkably constant for the coins of the emperors from Augustus to Claudius inclusive, as is shown in Table LV. In this table the data for Period I are based on the new analyses, except for the coins of Vespasian, no specimens of which were analyzed in the present investigation, and the data for Period II are based on the "good" analyses. The figures in the third column of the table show the true zinc to copper ratios, and those in the fourth column the ratios of the proportion of zinc to the proportion of copper plus its principal associated impurities, in other words the ratio of zinc to copper of the average quality actually used in the manufacture of the
| Period | Emperor |

|
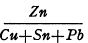
|
| I | Augustus | 0.285 | 0.283 |
| Caligula | 0.286 | 0.286 | |
| Claudius | 0.289 | 0.286 | |
| Nero | 0.249 | 0.248 | |
| Vespasian | 0.176 | 0.175 | |
| Nerva | 0.155 | 0.154 | |
| II | Trajan | 0.156 | 0.151 |
| Hadrian | 0.125 | 0.123 | |
| Antoninus Pius | 0.114 | 0.113 | |
| Marcus Aurelius and Lucius Verus | 0.071 | 0.064 | |
| Commodus | 0.051 | 0.046 |
What now needs to be explained is the progressive fall in the average zinc content of orichalcum coins in Period I after the reign of Claudius. One possible cause is that zinc minerals and ores were becoming increasingly scarce and costly due to the exhaustion of certain deposits, and that this led to the use of less zinc compound in the manufacture of orichalcum. But this is not the only possible cause. Another, and perhaps more likely one, is that worn coins from earlier reigns were now being remelted for the manufacture of metal for new coins. If worn sestertii, dupondii and asses were melted together for this purpose the resulting alloy would, of course, have a lower zinc content. Even if only worn sestertii and dupondii were remelted the resulting alloy would have a lower zinc content than the worn coins. On melting brass there is a preferential loss of zinc due to volatilization and oxidation. This is a well known phenomenon that has received considerable study. The average loss of zinc that commonly occurs in melting and pouring common yellow brass in modern American foundry practice is about six per cent of that originally present. 100 The further losses on subsequent treatment, such as annealing, amount to about four per cent, for a total of about ten per cent. With alloys of lower zinc content, similar to orichalcum, the percentage loss is less with the same time and temperature of exposure. 101 However, with the smaller scale operations and cruder methods of the ancient founders the loss with alloys of lower zinc content may well have equalled or exceeded that encountered in modern practice with alloys of higher zinc content. On the assumption that the loss would have been about ten per cent on remelting worn orichalcum coins, it is interesting to calculate what effect this would have had on the ratio of zinc to copper if coins of emperors prior to Nero were remelted to furnish metal for new coins. If the ratio of zinc to copper in the worn coins was about 0.285, a loss of ten per cent would reduce this ratio to about 0.257, and if the loss in weight were compensated by the addition of copper, the ratio would become about 0.250. From Table LV it will be seen that both figures are rather close to the average ratio for the coins of Nero. Hence it is entirely possible that much of the orichalcum for the coins of Nero and subsequent emperors in the latter part of Period I was obtained by remelting worn coins from previous reigns.
In view of the great volume of the orichalcum coinage of Trajan, Hadrian and Antoninus Pius, it may be doubted that this practice was sufficient to supply metal for all the orichalcum coins issued in the first part of Period II. Probably some new orichalcum was made during the reigns of these emperors, and this supply of new metal supplemented the supply of secondary metal obtained by remelting worn coins. That considerable secondary metal was used is indicated by the marked increase in the average tin and lead content of the coins of Trajan, which is evident from the ratios listed in Table LVI. This increase shows that some bronze was being added at some point in the manufacturing process, most likely in the remelting of coins. Possibly worn Roman colonial bronze coins were now being included in small proportion among those remelted. The wide range in the zinc content of the sestertii and dupondii of Trajan (Table XXXIX) indicates the use of both new and secondary metal for orichalcum coinage during his reign. The same seems true for the coins of Hadrian and to a lesser extent for those of Antoninus Pius. After the reign of Antoninus Pius the sudden decrease in the average zinc content
| Period | Emperor |
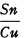
|

|

|
| I | Augustus | 0.0029 | 0.0035 | 0.0064 |
| Caligula | 0.0006 | 0.0009 | 0.0015 | |
| Claudius | 0.0013 | 0.0063 | 0.0076 | |
| Nero | 0.0000 | 0.0018 | 0.0018 | |
| Nerva | 0.0064 | 0.0029 | 0.0093 | |
| II | Trajan | 0.0202 | 0.0129 | 0.0331 |
| Hadrian | 0.0100 | 0.0055 | 0.0155 | |
| Antoninus Pius | 0.0119 | 0.0016 | 0.0135 | |
| Marcus Aurelius and Lucius Verus | 0.0560 | 0.0402 | 0.0962 | |
| Commodus | 0.0511 | 0.0617 | 0.1128 |
The increasing addition of poor quality metal to the coinage alloy in the latter part of Period II is indicated by the progressive increase in the total proportions of arsenic and sulfur, as is shown by Table LVII which lists the percentages of arsenic and sulfur in all the coins from Tiberius to Commodus inclusive in which both these elements were determined. Especially significant is the general absence of sulfur from coins of Period I and the general progressive increase in the proportion of this element in coins of Period II. This appears to mean that the copper entering into the alloy of the earlier coins was made from oxidized or surface ores, and that an increasing proportion of the copper that entered into the alloy of later coins was produced from the deeper sulfide ores. It also appears to indicate a growing scarcity of copper, for the oxidized ores were much easier to work. Recourse to the sulfide ores would only have come after the supply of oxidized ores had been practically exhausted. This may be still another cause for the extensive reworking of metal for the production of the minor coinage after the time of Antoninus Pius.
| Example No. | Date a.d. | Arsenic % | Sulfur % | Total % |
| 1 | 22–23 | none | none | none |
| 2 | 37–38 | 0.02 | none | 0.02 |
| 3 | 39–40 | none | none | none |
| 4 | 39–40 | none | none | none |
| 5 | 41 | 0.04 | none | 0.04 |
| 6 | 41 | 0.02 | none | 0.02 |
| 7 | 64–66 | 0.05 | none | 0.05 |
| 8 | 96–98 | 0.02 | none | 0.02 |
| 9 | 112–114 | none | none | none |
| 10 | 116–117 | 0.03 | 0.08 | 0.11 |
| 11 | 141+ | 0.06 | 0.12 | 0.18 |
| 12 | 154–155 | 0.10 | 0.17 | 0.27 |
| 13 | 154–155 | 0.12 | 0.21 | 0.33 |
| 14 | 161–162 | 0.21 | 0.17 | 0.38 |
| 15 | 162–163 | 0.08 | 0.28 | 0.36 |
| 16 | 179 | 0.13 | 0.32 | 0.45 |
Although our information about the composition of the sestertii and dupondii issued by the successors of Commodus is fragmentary (Tables XLIV to XLIX inclusive), it clearly indicates a still greater use of miscellaneous secondary metal. Especially significant are the high proportions of lead in many of the coins (Table XLIV; Table XLVII, Nos. 1, 2, 3; Table XLVIII, No. 3; Table XLIX). Since bronze of high lead content was commonly used for the late local coins of the empire, and for other purposes as well in the same general period, the high proportions of lead in these late sestertii and dupondii may have been the result of including worn local coins, as well as miscellaneous high lead bronze, in the metal remelted for coinage. The great variation in the composition of late sestertii and dupondii is a strong indication of the use of miscellaneous metal of varied composition for the production of the coinage alloy.
Economic factors may have also contributed to the progressive decrease in the zinc content of orichalcum coins after the reign of Claudius. Because of the additional raw material and the additional smelting operations required, orichalcum, from the beginning, must have been always a more costly coinage alloy than unalloyed copper. Perhaps the double value of the dupondius in relation to the as of the same weight represents the approximate initial ratio of the cost of orichalcum to that of copper. As nearby zinc ore deposits were exhausted and more distant sources had to be utilized, this ratio would become higher, unless the proportion of zinc ore or mineral used in the manufacture of the alloy was decreased with the resulting production of orichalcum of lower zinc content. This would account for the composition of certain orichalcum coins of low zinc content issued in the first part of Period II (e.g., Nos. 4 and 5 of Table XLI), for these coins also have a very low tin and lead content which indicates that they were made from newly manufactured orichalcum rather than from secondary metal of any kind.
The extensive use of secondary metal for sestertii and dupondii in the latter part of Period II and especially in the first half of the third century may have been caused in large part by the growing debasement of the silver coinage. Though it seems generally agreed that sestertii and dupondii were token coins, they had, because of their much greater weight, a much larger intrinsic metal value compared to the precious metals than most modern token coins made of base alloys. As the Roman silver coinage became more debased the intrinsic metal value of the sestertii and dupondii approached closer and closer to their nominal value. Under these circumstances there was a strong inducement to strike them from cheap secondary or scrap metal rather than from a more costly newly manufactured alloy.
| 94 |
Grant, M., From Imperium to Auctoritas (Cambridge, 1946), p. 88.
|
| 95 |
Natural History, Book XXXIV, sec. 4.
|
| 96 |
Op.cit., Book XXXIV, secs. 100–103.
|
| 97 |
Materia Medica, Book V, Chapters 84–85.
|
| 98 |
Forbes, R. J., Metallurgy in Antiquity (Leiden, 1950), p. 277.
|
| 99 |
Materia Medica, Book V, Chapter 85.
|
| 100 |
Bassett, W. H., Journal of Industrial and Engineering Chemistry, IV (1912), pp. 164–165.
|
| 101 |
Johnston, J., Journal of the American Institute of Metals, XII (1918), pp. 15–26.
|
In addition to the orichalcum coins which have been analyzed, about fifty other metal objects excavated at various sites both within and beyond the boundaries of the Roman Empire have been found on analysis to be composed of alloys more or less similar to those of the coins in composition. Many of these objects are of uncertain period, some are very probably of medieval origin, and some can be dated with various degrees of certainty to the general period of the Roman Empire. Göbel 102 was the first to analyze a number of objects of this general class. The objects he analyzed were found in the course of excavations and explorations in 1839, at various sites in the Russian Baltic provinces of that time. Archaeological evidence indicated that these sites dated from the ninth to the eleventh centuries a.d. However, on the basis of his analyses, Göbel advanced the theory that the objects themselves were of Roman origin. He suggested the following alternate possibilities:
On comparing the composition of these objects with that of some orichalcum coins, Göbel concluded that the alloys were very similar. However, he had for this comparison only a few imperfect analyses of such coins. In fact the alloys are not closely similar in composition, for some very significant differences exist. This may be illustrated by his analyses of parts of two balances, which he particularly considered to be Roman, not only on the basis of the composition of the metal but also on the basis of their form and the weight system used for one of them. The results of his analyses are shown in Table LVIII. Though the range of the proportions of zinc fall well within the range for orichalcum, the proportions of tin in three of the objects and the proportion of lead in the other one are much too high for orichalcum containing such proportions of zinc. As is apparent from Tables XXXI to XXXV inclusive, orichalcum that contains twenty per cent or more of zinc contains very little tin. Even the object in which only a trace of tin was present contained much more lead than is found in orichalcum coins of similar zinc content, as may be seen from Tables XXXVI to XL inclusive. The composition of these objects therefore differs from that of orichalcum, at least the kind of orichalcum used for coinage, and, as previously remarked, it is very probable that the Romans manufactured the alloy only for this purpose. Hence the theory of Göbel appears to have no sound basis, and a post-Roman date for the objects found in the Baltic provinces seems very probable.
| Balance No. | Part | Copper % | Zinc % | Tin % | Lead % | Total % |
| I | Beam | 76.50 | 20.30 | 2.45 | trace | 99.25 |
| Pan | 76.45 | 20.03 | 3.51 | trace | 99.99 | |
| II | Pan | 79.45 | 16.95 | 2.25 | 1.31 | 99.96 |
| Weight | 80.95 | 13.86 | trace | 5.25 | 100.06 |
Still other differences, such as the proportions of certain impurities such as iron, may serve to distinguish orichalcum from copper-zinc alloys made in medieval times. This may be illustrated by the analyses, published by Wocel, 103 of two small objects found in Bohemia and believed to date from near the end of the first millenium a.d. The results of these analyses are shown in Table LIX. Object No. 1 not only contained much higher proportions of tin and lead than is present in orichalcum containing over twenty per cent of zinc, but also contained a much higher proportion of iron than orichalcum of any zinc content, as may be seen from the proportions of iron listed in Tables X and XVIII. Object No. 2 contained higher proportions of tin and lead than is normally present in orichalcum containing nearly ten per cent of zinc, and an even higher proportion of iron than No. 1. By reason of such differences, medieval objects composed of copper-zinc alloys may usually be distinguished from Roman objects of similar zinc content by chemical analysis.
| Object No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Total % |
| 1 | 68.69 | 20.89 | 3.80 | 4.02 | 2.31 | 99.71 |
| 2 | 79.85 | 9.95 | 2.65 | 4.20 | 3.53 | 100.18 |
Listed in Table LX are the available analyses of those orichalcum objects other than coins that can be ascribed to the Roman Imperial period on the basis of both archaeological evidence and chemical composition. Nos. 1, 9 and 10 were analyzed by Fellenberg, 104 Nos. 2 and 3 by Gowland, 105 Nos. 5 and 6 by Church 106 and the remainder by Bibra. 107 For some of the objects, i.e., Nos. 2, 3, 5, 6 and 10, the archaeological evidence for a Roman date is stronger than for the others. No. 10 is particularly interesting in this respect. This object had the form of a thin curved rectangular plate about six inches long and half as wide, and was provided with holes at the four corners, which, together with its inscription, indicated that it was a name plate. It was found near Basel-Augst, Switzerland, by a farmer, who nailed it to his wagon as an ornament. Because of the apparent antiquity of the object and the curious inscription on it, the farmer brought it to the attention of a local manufacturer, who acquired it. A short account of this object and the circumstances of its discovery was published afterwards by Professor Roth. 108
In spite of the layers of green corrosion products and some hammer marks, the inscription on the convex side was easily legible
and complete. It consisted of three lines of capital letters as follows:
DEO INVICTO
TYPVM AVROCHALCVM
SOLIS.
This may be translated as follows: (To the invincible God an orichalcum figure of Sol). There can be little doubt that the deity referred to is Mithras. It seems not unlikely that the figure was made of the same alloy as its name plate. This plate is the largest orichalcum object so far analyzed, and of course the statue or statuette was much larger.
| Object No. | Copper % | Zinc % | Tin % | Lead % | Other Components % | Total % |
| 1 | 75.07 | 24.45 | 0.20 | none | 0.28 | 100.00 |
| 2 | 80.42 | 18.77 | none | 0.09 | 0.62 | 99.90 |
| 3 | 82.31 | 17.11 | none | 0.08 | 0.45 | 99.95 |
| 4 | 82.01 | 15.30 | 1.79 | 0.80 | 0.10 | 100.00 |
| 5 | 84.27 | 14.70 | 2.36 | — | — | 101.33 |
| 6 | 84.90 | 13.00 | 1.03 | 1.07 | — | 100.00 |
| 7 | 84.45 | 12.31 | 1.72 | 1.44 | 0.08 | 100.00 |
| 8 | 86.95 | 11.03 | 1.35 | 0.31 | 0.36 | 100.00 |
| 9 | 87.07 | 10.87 | 0.91 | 0.75 | 0.40 | 100.00 |
| 10 | 85.96 | 10.61 | 2.40 | — | 1.03 | 100.00 |
| 11 | 85.94 | 9.31 | 1.50 | 2.03 | 1.22 | 100.00 |
| 12 | 87.28 | 8.22 | 2.00 | 1.70 | 0.80 | 100.00 |
In view of the apparent monopoly that the Roman state held on the manufacture of orichalcum, it follows that orichalcum coins were probably the immediate source of the metal for these miscellaneous objects. From Table LX it will be seen that the proportions of the main components of the alloys vary in the same way as in orichalcum coins, for the objects of highest zinc content (Nos. 1, 2 and 3) contain very little tin and lead, those of intermediate zinc content contain appreciable proportions of tin and lead, and those of lowest zinc content (Nos. 11 and 12) contain the highest total proportions of tin and lead. By comparing the figures of Table LX with those of Tables XXXI to XLI inclusive it will be seen that the composition of most of these objects is close to that of certain orichalcum coins of similar zinc content. These similarities tend to support the theory that orichalcum coins were the source of the metal for these objects. Though no weights are given, most of them apparently weighed less than a sestertius and could have been made from single coins. If objects of this sort were fashioned from coins by simple cold working, their composition should be identical with that of the coins from which they were made, but if their production involved the remelting of coins, some differences in composition might be expected because of probable loss of zinc through volatilization and oxidation.
Though no dates have been assigned to any of the objects listed in Table LX, it should be possible, because of the chronological changes in the composition of orichalcum, to make some rough estimates of their dates. For the same reason it should be possible to estimate from the results of analyses the dates of orichalcum coins that are illegible because of wear or corrosion. This might be especially useful for coins of this sort found in excavations when the dates of the strata in which they are found or the dates of associated objects need to be determined. Since the dates of such coins can be estimated more closely than the dates of the objects, this problem will be considered first.
Although the average zinc, tin, and lead content of the coins issued in the successive reigns undoubtedly vary in a systematic way chronologically, as is shown in Table LI, these averages cannot be used to date individual illegible coins because of the considerable range in the proportions of these metals in the coins issued in any given reign. The only feasible way to estimate the date of a single coin would seem to be to establish a series of composition patterns, or categories of composition, each of which will extend over several reigns and then to compare the composition of the given coin with this series until a match is obtained. In Table LXI is shown a series of categories of composition for orichalcum and zinc bronze sestertii and dupondii derived from the analyses listed in Tables XXXI to XLIX inclusive, together with the corresponding date ranges. Not covered by these categories and ranges are the very few coins of uncertain date or distinctly atypical composition. As would be expected, many of these date ranges overlap each other. They also differ much in length so that the possibility of dating a given coin closely is very dependent on its composition. For example, if it falls into Category I, a fairly close estimate is possible, but not if its composition falls into Category VII. One possible way by which some of these date ranges could be narrowed would be to include also the total proportion of arsenic and sulfur as an index of date of manufacture. As is shown in Table LVII, significant total proportions of these elements appear to occur only in the orichalcum coins of Period II. Furthermore, their total proportion appears to increase chronologically throughout this period. If a coin falling into Category XV with its wide date range from a.d. 98 to a.d. 192 were also analyzed for arsenic and sulfur, the results should narrow this range considerably, for the presence of little or no arsenic and sulfur would indicate a date near the beginning of this range, the
| Category | Zinc Content % | Total Tin and Lead Content % | Possible Date Range |
| I | 25.0 and over | Under 0.5 | 37 a.d. to 54 a.d. |
| II | 24.9 to 22.5 | Under 0.5 | 23 b.c. to 54 a.d. |
| III | 22.4 to 20.0 | Under 0.5 | 23 b.c. to 68 a.d. |
| IV | 22.4 to 20.0 | Over 0.5 | 23 b.c. to 54 a.d. |
| V | 19.9 to 17.5 | Under 1.0 | 37 a.d. to 117 a.d. |
| VI | 19.9 to 17.5 | Over 1.0 | 54 a.d. to 68 a.d. |
| VII | 17.4 to 15.0 | Under 1.0 | 23 b.c. to 138 a.d. |
| VIII | 17.4 to 15.0 | Over 1.0 | 69 a.d. to 117 a.d. |
| IX | 14.9 to 12.5 | Under 1.0 | 69 a.d. to 161 a.d. |
| X | 14.9 to 12.5 | Over 1.0 | 96 a.d. to 138 a.d. |
| XI | 12.4 to 10.0 | Under 0.5 | 138 a.d. to 180 a.d. |
| XII | 12.4 to 10.0 | 0.5 to 5.0 | 81 a.d. to 161 a.d. |
| XIII | 12.4 to 10.0 | Over 5.0 | 161 a.d. to 192 a.d. |
| XIV | 9.9 to 7.5 | Under 1.0 | 138 a.d. to 161 a.d. |
| XV | 9.9 to 7.5 | 1.0 to 5.0 | 98 a.d. to 192 a.d. |
| XVI | 9.9 to 7.5 | Over 5.0 | 192 a.d. to 244 a.d. |
| XVII | 7.4 to 5.0 | Under 1.0 | 117 a.d. to 161 a.d. |
| XVIII | 7.4 to 5.0 | 1.0 to 5.0 | 117 a.d. to 192 a.d. |
| XIX | 7.4 to 5.0 | 5.1 to 10.0 | 161 a.d. to 217 a.d. |
| XX | 7.4 to 5.0 | 10.1 to 15.0 | 161 a.d. to 192 a.d. |
| XXI | 7.4 to 5.0 | Over 15.0 | 192 a.d. to 249 a.d. |
| XXII | Under 5.0 | Under 10.0 | 161 a.d. to 192 a.d. |
| XXIII | Under 5.0 | Over 10.0 | 161 a.d. to 244 a.d. |
Shown in Table LXII are estimates, from the categories and ranges of Table LXI, of date limits for the manufacture of the objects of Table LX. These estimates involve three assumptions, which are, that orichalcum coins were the immediate source of the metal for these objects, that the objects were made from coins without significant change in the composition of the alloys and that each of the objects was made during, or very shortly after, the period of issue of the coin or coins from which it was made. The very probable validity of the first assumption, on which the rest depend, has already been discussed. As to the validity of the second assumption, the small size of most of the objects would make it seem likely that they were fashioned from coins largely by cold working, which would not have caused any change of composition. Even if some annealing had been necessary, the change in composition probably would not have been significant. But Object No. 10, because of its size, could not have been made without melting coins together, and this would have caused some loss of zinc. However, a calculation shows that even if the original coins had lost ten per cent of their zinc content on fusion, the composition of the coins would still fall in the same category as the composition of the object. The same is true for more than half the other objects. Even for those in which a change of category would occur, the change would usually not shift either the category or the date limits very much. For example, if the manufacture of Object No. 1 involved the fusion of a coin or coins and a ten per cent loss of zinc occurred, a calculation shows that the original coin or coins would have contained about 26.5% zinc, which would place them in Category I with the limits of a.d. 37 to a.d. 54 instead of Category II with the limits of 23 b.c. to a.d. 54. In other words only the lower limit would be changed. Likewise for Object No. 2, for which the shift would be from Category V to Category III with a change in the lower limit but not the upper limit. No technical arguments can be advanced to support the validity of the third assumption. It merely rests on considerations of probability, for it seems inherently much more probable that objects would have been fashioned from particular coins at a time when they were being abundantly circulated and were freely available, rather than at some later time. However, many of the upper limits of Table LXII allow a considerable margin for the possibility that objects made at a given time were in fact made from coins that were no longer being issued. For example, Object No. 12 could have been made as late as the reign of Commodus from a coin
| Object No. | Category of Composition | Lowest Possible Limit of Date of Manufacture | Probable Upper Limit of Date of Manufacture |
| 1 | II | 23 b.c. | 54 a.d. |
| 2 | V | 37 a.d. | 68 a.d. |
| 3 | VII | 23 b.c. | 138 a.d. |
| 4 | VIII | 69 a.d. | 117 a.d. |
| 5 | X | 96 a.d. | 138 a.d. |
| 6 | X | 96 a.d. | 138 a.d. |
| 7 | XII | 81 a.d. | 161 a.d. |
| 8 | XII | 81 a.d. | 161 a.d. |
| 9 | XII | 81 a.d. | 161 a.d. |
| 10 | XII | 81 a.d. | 161 a.d. |
| 11 | XV | 98 a.d. | 192 a.d. |
| 12 | XV | 98 a.d. | 192 a.d. |
In any investigation of the composition of orichalcum objects it should be realized that there are in existence forgeries of ancient objects composed of copper alloys containing zinc. Otto 109 has shown that various German museums possess a number of forgeries of prehistoric bronze objects composed of such alloys. His analyses show that most of them contain from ten to fifteen per cent zinc in addition to various proportions of tin and lead. Relative to the proportions of zinc the total proportions of tin and lead are usually so high that the composition of the alloys is clearly very different from that of ancient orichalcum or zinc bronze. There can be no doubt that these forgeries were cast from modern zinc bronzes.
Shown in Table LXIII are the results of analyses of various modern forgeries which were alleged to be ancient objects found in the Mediterranean region. All except No. 5 were analyzed by the author. The genuineness of Nos. 1 through 4 was questioned partly on the basis of style but chiefly because of the presence of suspicuously thin layers of corrosion products on the metal. Examination showed that the layer on each of these objects was not only abnormally thin for an object of such alleged antiquity but that the metal immediately
| Object No. | Copper % | Zinc % | Tin % | Lead % | Iron % | Nickel % | Total % |
| 1 | 69.07 | 23.53 | 1.90 | 4.64 | 0.64 | — | 99.78 |
| 2 | 78.83 | 11.73 | 5.04 | 3.24 | 0.56 | 0.38 | 99.78 |
| 3 | 83.25 | 4.87 | 7.36 | 4.40 | 0.15 | — | 100.03 |
| 4 | 68.67 | 15.34 | 10.33 | 4.45 | 1.00 | 0.16 | 99.95 |
| 5 | 81.75 | 10.5 | 5.89 | 1.7 | — | — | 99.8 |
It will be seen that the alloy of No. 1 is a leaded brass and not a bronze as alleged. Though the zinc content is that of early orichalcum, the proportions of tin and lead are much higher than in orichalcum containing a similar proportion of zinc. The alloy of No. 2 might be classed as a zinc bronze rather than a brass, but genuine Etruscan statuary bronze is a simple tin bronze containing very little lead and no more than traces of zinc. No. 3 was said to be an early Greek bronze, but genuine early Greek statuary bronze is a simple tin bronze very similar in composition to Etruscan statuary bronze. Furthermore, the metal of this object contained only traces of the various impurities usually found in appreciable proportions in ancient bronzes. The alloy of No. 4 might well be classed as a zinc bronze rather than a brass. This object was alleged to date from about a.d. 15, but analyses of genuine Roman statuary bronzes of the Imperial period show that they are always leaded tin bronzes containing very little zinc. Of course it might be argued that this was an exceptional object cast from orichalcum or ancient zinc bronze, but the analysis demolishes this argument since the alloy contains too little zinc and far too much tin and lead for orichalcum of the early part of the first century a.d. and too much zinc for ancient zinc bronze of later date. No. 5 was analyzed by Göbel, 110 who described the coin as follows:
Obv.: Head with inscription, C. Caesar Dictator.
Rev.: Laurel-wreath, inside which is inscription, veni, vidi, vici.
Göbel apparently did not suspect that this might be a forgery, but no other example of a coin of this type with its suspicious inscription is known, and the results of the analysis clearly show that it is a forgery. Although the composition of the alloy falls within the range of composition of some late orichalcum, it is far different in composition from orichalcum of the Roman Republican period, as may be seen by comparing the results of this analysis with those in Table IV. However, it is possible that the metal for this forgery was obtained by melting down some genuine late orichalcum coins. This appears to be the only forgery of a Roman orichalcum coin shown to be such by the analysis of the alloy. Probably analysis could be used to prove the falseness of other forgeries of orichalcum coins.
| 102 |
Göbel, F., Ueber den Einfluβ der Chemie auf die Ermittelung der Völker der Vorzeit oder Resultate der chemischen Untersuchung metallischen
Alterthümer inbesondere der in den Ostseegouvernements vorkommenden, Behufs der Ermittelung der Völker, von welchen sie abstammen (Erlangen, 1842), pp. 18–22, 32–34.
|
| 103 |
Wocel, J. E., Sitzungsberichte der kaiserlichen Akademie der Wissenschaften, Wien, Philosophisch-Historische Classe, XI (1853), pp. 723, 728.
|
| 104 |
Fellenberg, L. R. von, Mitteilungen der Naturforschenden Gesellschaft in Bern (1860), pp. 74–75; Ibid. (1861), p. 179; Ibid. (1863), p. 139.
|
| 105 |
Gowland, W., Journal of the Institute of Metals, VII (1912), p. 44.
|
| 106 |
Church, A. H., Journal of the Chemical Society, XVIII (1865), pp. 215–217.
|
| 107 |
Bibra, E. von, Die Bronzen und Kupferlegirungen der alten und ältesten Völher (Erlangen, 1869), pp. 70–71.
|
| 108 |
Roth, K. L., Anzeiger für schweizerische Geschichte und Alterthumskunde (March, i860), p. 85.
|
| 109 |
Otto, H., Wissenschaftliche Zeitschrift der Martin-Luther-Universitäi Halle-Wittenberg, VII (1957), pp. 203–230.
|
| 110 |
Göbel, F., Ueber den Einfluβ der Chemie auf die Ermittelung der Völker der Vorzeit oder Resultate der chemischen Untersuchung metallischen
Alterthümer inbesondere der in den Ostseegouvernements vorkommenden, Behufs der Ermittelung der Völker, von welchen sie abstammen (Erlangen, 1842), p. 30.
|